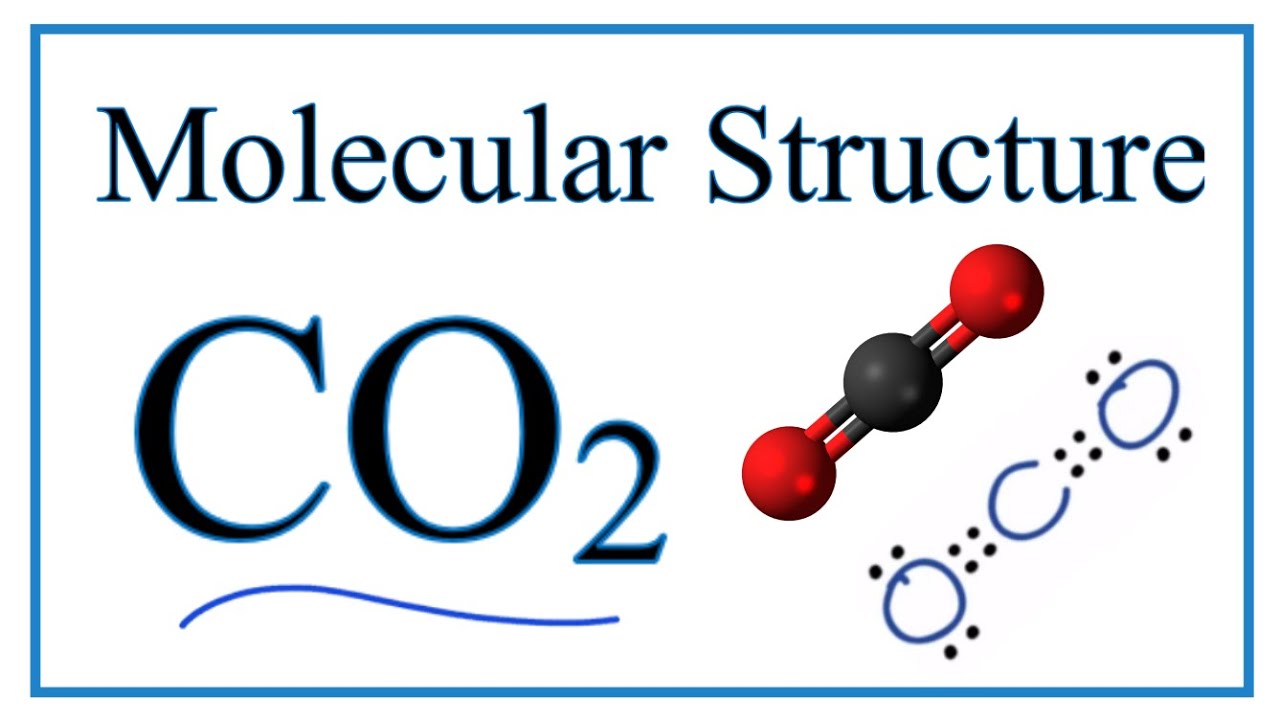
A carbon dioxide molecule is covalently doubly linked to two oxygen atoms in a carbon dioxide molecule. It is found as a trace gas in the Earth’s atmosphere. The current concentration is around 0.04 percent (412 ppm) by volume, up from 280 ppm before the industrial revolution.
 CO2 Molecule:
CO2 Molecule:
A carbon atom is covalently bonded to two oxygen atoms in a carbon dioxide molecule. It exists as a trace gas in the Earth’s atmosphere.The current concentration is roughly 0.04 percent (412 parts per million) by volume, up from 280 parts per million prior to the industrial revolution.
Natural sources include volcanoes, forest fires, hot springs, and geysers, and it is liberated from carbonate rocks by dissolving in water and acids. Because carbon dioxide is soluble in water, it can be found in groundwater, rivers and lakes, ice caps, glaciers, and the ocean.
Petroleum and natural gas reserves contain it. Carbon dioxide has a pungent, acidic odour and tastes like soda water in the mouth. At ordinary concentrations, it is, however, odourless.
Carbon dioxide is the primary source of accessible carbon in the carbon cycle, and its concentration in the pre-industrial atmosphere has been regulated by photosynthetic organisms and geological events since late in the Precambrian.Photosynthesis is a process in which plants, algae, and cyanobacteria use energy from sunshine to synthesize carbohydrates from carbon dioxide and water, producing oxygen as a waste product.
When aerobic organisms digest organic molecules for energy, oxygen is consumed and CO2 is generated as waste. Because plants require CO2 for photosynthesis and humans and animals rely on plants for nutrition, CO2 is essential for life on Earth.
It is returned to the water by fish gills, and it is returned to the air by air-breathing terrestrial organisms, including humans. In the manufacture of bread, beer, and wine, carbon dioxide is created by the breakdown of organic components and the fermentation of carbohydrates.
It is produced from the combustion of wood, peat, and other organic materials, as well as fossil fuels such as coal, petroleum, and natural gas. It’s an unwelcome consequence of a number of large-scale oxidation processes, including the production of acrylic acid (which produces over 5 million tonnes per year).
It’s a versatile industrial material that’s used in welding and fire extinguishers, as a pressurising gas in air guns and oil recovery, as a chemical feedstock, and as a supercritical fluid solvent in coffee decaffeination and supercritical drying, among other things.
It is used to give effervescence to drinking water and carbonated beverages such as beer and sparkling wine. Dry ice is a frozen solid form of CO2 that is used as a refrigerant and abrasive in dry-ice blasting. It is utilised in the production of fuels and chemicals as a raw ingredient.
Carbon dioxide is the most common long-lived greenhouse gas in the Earth’s atmosphere. Since the Industrial Revolution, anthropogenic emissions, primarily from the use of fossil fuels and deforestation, have dramatically increased their concentration in the atmosphere, resulting in global warming.Because carbon dioxide dissolves in water and forms carbonic acid, it contributes to ocean acidification.
 History:
History:
The first gas to be classified as a distinct substance was carbon dioxide. Jan van Helmont, a Flemish chemist, discovered that when he burned charcoal in a sealed container, the mass of the resulting ash was substantially less than that of the originating charcoal in around 1640. The rest of the charcoal, he believed, had been transfigured into an invisible substance he referred to as a “gas” or “wild spirit” (spiritus sylvestris).
In the 1750s, Scottish physician Joseph Black investigated the properties of carbon dioxide further. He discovered that heating limestone (calcium carbonate) or treating it with acids produced a gas he named “fixed air.” He saw that the fixed air was denser than air and could not support flames or animal life.
When limewater (a copious aqueous phase of calcium hydroxide) was bubbled through, it precipitated calcium carbonate, according to Black. He exploited this event to show how animal breathing and microbial fermentation both produce carbon dioxide.
In 1772, English chemist Joseph Priestley published Impregnating Water with Fixed Air, describing a method for creating carbon dioxide by dripping sulfuric acid (or oil of vitriol as Priestley understood it) over chalk and forcing the gas to dissolve by swirling a bowl of water in contact with the gas.
Humphry Davy and Michael Faraday were the first to liquefy carbon dioxide (at high pressures) in 1823. Adrien-Jean-Pierre Thilorier, a French inventor, was the first to describe solid carbon dioxide (dry ice), when he opened a pressurized container of liquid carbon dioxide in 1835, only to discover that the rapid evaporation of the liquid resulted in a “snow” of solid CO2.
 Biological role:
Biological role:
Carbon dioxide is a byproduct of cellular respiration, which occurs when organisms use oxygen to break down carbohydrates, lipids, and amino acids for energy. All plants, algae, and animals, as well as aerobic fungi and bacteria, are included.
Carbon dioxide passes through the blood from the body tissue to the skin (e.g., amphibians) or gills (e.g., fish), where it breaks in the water, or to the lungs, where it is expelled invertebrates. Plants can take more co2 from the atmosphere during active photosynthesis than they release during respiration.
Summary:
Carbon dioxide is found as a trace gas in the Earth’s atmosphere. Natural sources include volcanoes, forest fires, hot springs, and geysers. CO2 is essential for life on Earth since plants need it for photosynthesis and humans rely on it for nutrition. In the Earth’s atmosphere, carbon dioxide is the most prevalent long-lived greenhouse gas.Anthropogenic emissions – primarily from the burning of fossil fuels and deforestation – have boosted their concentration in the atmosphere dramatically.
 Photosynthesis and carbon fixation
Photosynthesis and carbon fixation
Carbon fixation is a biological process in which plants, algae, and (cyanobacteria) convert atmospheric carbon dioxide into energy-rich organic molecules like glucose, allowing them to produce their food through photosynthesis. Photosynthesis produces sugars from carbon dioxide and water, which can be used to make various organic molecules, as well as oxygen as a byproduct.
RuBisCO stands for ribulose-1,5-bisphosphate carboxylase oxygenase, which is engaged in the first major step of carbon fixation, the creation of two molecules of 3-phosphoglycerate from CO2 and ribulose bisphosphate.
Phototrophs utilize photosynthetic products as internal food sources and as raw materials for the manufacture of more complex organic compounds like polysaccharides, nucleic acids, and proteins. These are employed for their growth as well as to form the foundation of food chains and webs that feed other organisms, including humans.
The coccolithophores, which are significant phototrophs, produce hard calcium carbonate scales. Emiliania huxleyi is a globally prominent coccolithophore whose calcite scales have formed the basis of numerous sedimentary rocks, including limestone, where previously atmospheric carbon can be preserved over geological timescales.
Because plants and algae that utilize C3 photosynthesis also exhale CO2 during respiration, the majority of plants and algae that employ C3 photosynthesis are only net absorbers during the day.
A mature forest will produce as much CO2 through respiration and decomposition of expired specimens (e.g., fallen branches) as is required in photosynthesis in growing plants, even though a developing forest absorbs many tonnes of CO2 each year.
Despite the popular belief that mature forests are carbon-neutral, they can continue to accumulate carbon and serve as vital carbon sinks, assisting in the maintenance of the Earth’s carbon balance. Furthermore, and significantly for life on Earth, phytoplankton photosynthesis consumes dissolved CO2 in the upper water, promoting CO2 absorption from the atmosphere.
 How to draw Carbon dioxide Lewis Structure?
How to draw Carbon dioxide Lewis Structure?
Two oxygen atoms and one carbon atom are joined by a double bond in the CO2 Lewis structure, whereas carbon is the center element with no lone pair.
In the CO2 lewis dot structure, however, each oxygen has two lone pairs. A Lewis diagram shows how electrons in a molecule are organized around particular atoms. Let’s have a look at how to draw a CO2 Lewis structure in a few easy steps.
 1. Count total valence electron in CO2
1. Count total valence electron in CO2
The first step is to figure out how many valence electrons there are in it. The outer shell electrons present around carbon and oxygen that can participate in the creation of chemical bonds are shown by the valence electrons of CO2.
In the Lewis diagram, the valence electrons of Carbon and Oxygen are depicted as dots. A periodic table is required to determine the valence electron of carbon and oxygen.
Looking at the periodic table, we can see that carbon belongs to 14 groups and oxygen to the 16th. As a result, carbon has four valence electrons and oxygen has six.
Oxygen’s valence electron is 6 [Oxygen’s periodic group is 16].
Carbon’s valence electron is 4 [Carbon’s periodic group is 14].
Total number of valence electrons available to draw the Lewis structure of CO2 = 4 + 2*6 = 16
 2. Find the least electronegative atom and placed it at center
2. Find the least electronegative atom and placed it at center
Now we must determine whether an atom (Carbon or oxygen) has the lowest electronegativity, and then place that atom in Lewis’ diagram’s center. The periodic table shows that electronegativity increases from left to right.
Carbon is in the 14th group on the periodic table, which is on the left, and oxygen is in the 16th group, which is on the right. Because carbon is less electronegative than oxygen, it should be in the center of the Lewis diagram, with oxygen evenly dispersed around it.
 3. A single bond connects carbon and oxygen.
3. A single bond connects carbon and oxygen.
In the third phase, we’ll start drawing the Lewis structure of CO2 by using a single bond to connect the outer atom (Oxygen) to the central atom (Carbon).
Looking at the diagram above, we can see that two single bonds with four electrons are utilized. As a result, we used 4 electrons out of a total of 16 valence electrons to draw the Lewis structure of CO2.
Valence electrons (16 – 4) = 12
 4. Remaining valence electrons were placed around the outer atom.
4. Remaining valence electrons were placed around the outer atom.
Because we only have 12 valence electrons left, we must first position them around the outer atom (Oxygen) to complete the octet rule. “According to the octet rule, an atom is most stable when it has eight electrons in its valence shell.”
As a result, oxygen requires 8 electrons to enter the stable zone. As a result, complete the octet shell of oxygen by placing the remaining valence electron around it first.
So, look at the diagram above to see how many valence electrons we’ve utilized so far and how many are still available. Because each oxygen atom has 8 electrons (6 dot electrons + 2 electrons in a single bond), oxygen may safely complete its octets.
16 valence electrons are employed in the preceding diagram (6 on each oxygen atom Plus 4 electrons in the form of two single bonds).
 5. Complete the core atom octet:
5. Complete the core atom octet:
This is the last stage in finishing the CO2 Lewis dot structure. For stability, we must complete the core atom (Carbon) octet in this step. Because carbon requires 8 electrons to complete its octet shell, it now contains just four electrons (two single bonds) and needs four more to complete it.
We also don’t have any extra valence electrons to complete the carbon octet. So, to solve this difficulty, we’ll use oxygen lone pair electrons. Take two lone pair electrons from each oxygen and make a covalent link with them.
Examine the CO2 lewis dot structure above to check if the atoms of CO2 have completed their octet. Yes, both carbon and oxygen atoms have comfortably completed their octet rule, as each possesses 8 electrons in the outermost shell.
 6. Use a formal charge notion to check for stability.
6. Use a formal charge notion to check for stability.
Simply use the formal charge notion to assess the stability of the CO2 Lewis structure. "The smaller the formal charge on atoms, the more stable the Lewis diagram."Applications:
Carbon dioxide is used in the food business, the oil industry, and the chemical industry. The chemical has a variety of commercial uses, but one of the most important is in the production of carbonated beverages; it is responsible for the sparkle in soda water, beer, and sparkling wine.
Summary:
Carbon fixation is a biological process in which plants, algae, and (cyanobacteria) convert atmospheric carbon dioxide into energy-rich organic molecules like glucose. Photosynthesis produces sugars from carbon dioxide and water, which can be used to make various organic molecules, as well as oxygen as a byproduct. Using the periodic table, we can see that carbon belongs to 14 groups and oxygen to the 16th.
 Precursor to chemicals
Precursor to chemicals
Carbon dioxide is mostly used in the chemical industry to produce urea, with a small amount being used to produce methanol and a number of other compounds. Several carboxylic acid derivatives, such as sodium salicylate, are made using the Kolbe-Schmitt process.
In addition to classic CO2 processes for chemical synthesis, electrochemical approaches are being examined at the research level. The use of renewable energy to produce CO2 fuels (such as methanol) is tempting because it could result in fuels that are easy to transport and use within present combustion technologies while releasing no net CO2.
 Agriculture
Agriculture
The presence of carbon dioxide is required for photosynthesis in plants. To maintain and accelerate plant development, greenhouse atmospheres may (and, if large enough, must) be enhanced with additional CO2.
Because very high concentrations of carbon dioxide (100 times atmospheric concentration or greater) can be harmful to animal life, raising the concentration to 10,000 ppm (1 percent) or higher for many hours will kill pests like whiteflies and spider mites in a greenhouse.
 Foods
Foods
Carbon dioxide is a food component that is used as a propellant and acidity control in the food industry. It is permitted for use in the EU, the US, Australia, and New Zealand (E number E290) (listed by its INS number 290).
Pop Rocks are a candy that is compressed with carbon dioxide gas at 4,000 kPa (40 bar; 580 psi). When you put it in your mouth, it melts (much like other hard candy) and pops, releasing the gas bubbles.
Because leavening agents produce carbon dioxide, the dough rises. When heated or subjected to acids, chemical leaveners like baking powder and baking soda release carbon dioxide, whereas baker’s yeast produces carbon dioxide by digesting carbohydrates within the dough.
 Beverages
Beverages
Carbon dioxide is used to manufacture carbonated soft drinks and soda water. Beer and sparkling wine have historically been carbonated through spontaneous fermentation, but many producers now carbonate these beverages using carbon dioxide collected during the fermentation process.
The most common technique of carbonation in bottled and kegged beer is recycled carbon dioxide. Except for British real ale, draught beer is generally transported from kegs in a cold room or basement to dispensing faucets on the bar using pressurised carbon dioxide, which is occasionally combined with nitrogen.
The flavour of soda water (and similar taste sensations in other carbonated beverages) is caused by dissolved carbon dioxide rather than the gas’s bursting bubbles. Carbonic anhydrase 4 converts carbonic acid to carbonic acid, which produces a sour taste and a tactile sensation from the dissolved carbon dioxide.
 Winemaking
Winemaking
Carbon dioxide in the form of dry ice is widely used during the cold soak phase of winemaking to quickly freeze clusters of grapes after picking to help avoid spontaneous fermentation by wild yeast.
The main advantage of using dry ice rather than water ice is that it cools the grapes without adding any additional water, which could reduce the sugar level in the grape must and, as a result, the alcohol concentration in the finished wine. Carbon dioxide is also used to create a hypoxic environment for carbonic maceration, which is the method used to make Beaujolais wine.
Carbon dioxide is sometimes used to keep wine bottles or other storage vessels like barrels from oxidising, but it has the disadvantage of dissolving in the wine, making a previously still wine slightly bubbly. As a result, expert winemakers use nitrogen or argon as an alternate gas for this operation.
 Inert gas
Inert gas
Carbon dioxide is one of the most commonly used compressed gases in pneumatic (pressurised gas) systems in portable pressure tools. Although it oxidises most metals in the welding arc, carbon dioxide is also used as a welding environment.
Despite evidence that carbon dioxide welds are more brittle than welds done in more inert atmospheres, carbon dioxide is widely used in the automotive industry. Because CO2 may react at these high temperatures, it is also referred to as MAG welding, or Metal Active Gas, when used for MIG welding.
It produces a hotter puddle than inert atmospheres, allowing for better flow qualities. However, this could be due to air processes at the puddle site. When welding, this is usually the opposite of the intended effect because it tends to embrittle the site, while it may not be an issue for typical mild steel welding when final ductility isn’t a major concern.
Carbon dioxide is used in many consumer products that require pressurised gas because it is inexpensive and nonflammable, and it transitions from gas to liquid at room temperature at an attainable pressure of approximately 60 bar (870 psi; 59 atm), allowing more carbon dioxide to fit in a given container than would be possible otherwise.
Life jackets often have pressurised carbon dioxide canisters for quick inflation. CO2 capsules can also be used to fill air guns, paintball markers/guns, inflate bicycle tyres, and make carbonated water.
Liquid carbon dioxide is employed in the supercritical drying of several food products and industrial materials, in the preparation of specimens for scanning electron microscopy, and the decaffeination of coffee beans, among other applications.
Summary:
Carbon dioxide can be hazardous to animal life at very high levels and should not be used for human health reasons. The use of renewable energy for the production of CO2 fuels (such as methanol) is appealing. Carbon dioxide is one of the most commonly used compressed gases in portable pressure tools’ pneumatic (pressurized gas) systems.
 Fire extinguisher
Fire extinguisher
By filling the region around the flame with carbon dioxide, flames can be extinguished. By moving the flame, it deprives it of oxygen rather than extinguishing it. A pressurised liquid carbon dioxide is contained in some fire extinguishers, notably those designed for electrical fires.
Carbon dioxide extinguishers are effective against small flammable liquid and electrical fires, but they are ineffective against larger combustible fires because they do not significantly cool the burning substances, and once the carbon dioxide has dissipated, they can catch fire when exposed to atmospheric oxygen. It’s most typical to find them in server rooms.
Carbon dioxide has also been used as an extinguishing chemical in stationary fire-fighting systems for localised threats and total flooding of a protected zone.
Carbon-dioxide systems for fire protection of ship holds and engine rooms are recognized by International Maritime Organization specifications. Multiple deaths have been linked to carbon-dioxide-based firefighting systems, as high levels of the gas can cause suffocation.An analysis of CO2 systems found 51 mishaps between 1975 and the study’s date (2000), resulting in 72 deaths and 145 injuries.
 Supercritical CO2 as a solvent
Supercritical CO2 as a solvent
Liquid carbon dioxide is used to decaffeinate coffee because it is a good solvent for many lipophilic chemical compounds. Carbon dioxide has piqued attention in the pharmaceutical and chemical processing industries as a safer alternative to traditional solvents such as organochlorides.
It’s also used by some dry cleaners for the same purpose (see green chemistry). Supercritical carbon dioxide is used in the production of a variety of aerogels due to its properties.
 Energy
Energy
For greater oil recovery, carbon dioxide is poured into or near producing oil wells under supercritical conditions, when it becomes miscible with the oil.This approach can increase original oil recovery by reducing residual oil saturation by 7% to 23% in addition to primary extraction.
When dissolved in subsurface crude oil, it acts as a pressurising agent and reduces viscosity and modifies surface chemistry, allowing the oil to flow more quickly through the reservoir and to the removal well. Large pipe networks convey carbon dioxide to injection locations in mature oil fields.
Carbon dioxide would be poured into the coal seam to displace methane, as opposed to conventional methods, which rely mostly on the removal of water (to reduce pressure) to get the coal seam to release its contained methane.
 Refrigerant
Refrigerant
Carbon dioxide is a crucial refrigerant in the food industry, where it is utilised in the shipping and storage of ice cream and other frozen foods in both liquid and solid form.Dry ice is a form of carbon dioxide that is utilised for small shipments when refrigeration isn’t possible.Regardless of air temperature, solid carbon dioxide is always below 78.5 °C (109.3 °F) at standard atmospheric pressure.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the adoption of dichlorodifluoromethane (R12, a chlorofluorocarbon (CFC) compound). Because 1,1,1,2-tetrafluoroethane (R134a, a hydrofluorocarbon (HFC) molecule) contributes more to climate change than CO2, CO2 may see a comeback.
CO2 is good for cooling, refrigeration, and heating because of its physical properties, which include a high volumetric cooling capacity. Due to the necessity to work at pressures of up to 130 bars, CO2 systems necessitate mechanically robust reservoirs and components that have already been designed for mass production in diverse sectors (1,900 psi; 13,000 kPa). For latitudes larger than 50 degrees, CO2 (R744) is more efficient than HFCs in car air cooling in more than 90% of all driving situations (e.g., R134a).
Its low GWP, non-ozone depleting, non-toxic, and non-flammable properties may make it the working fluid of the future, replacing current HFCs in autos, supermarkets, and heat pump water heaters, among other applications. Coca-Cola has deployed CO2-based beverage coolers, and the US Army is interested in CO2 refrigeration and heating technology.
 Minor uses
Minor uses
Carbon dioxide is used as the lasing medium in carbon-dioxide lasers, which are one of the oldest types of lasers. Carbon dioxide can be used to maintain the pH of swimming pools by continuously adding gas to the water and preventing it from rising.
One of the advantages of this is that it eliminates the need to handle (more hazardous) acids. It’s also commonly used in calcium reactors to lower the pH of the water as it passes through calcium carbonate, allowing the calcium carbonate to dissolve more easily into the water, where it’s needed by some corals to build their skeleton.
The primary coolant in the United Kingdom’s sophisticated gas-cooled reactor for nuclear power generation. Carbon dioxide induction is frequently used to euthanize laboratory research animals.
Animals can be given CO2 in one of two ways: they can be placed directly into a closed, prefilled chamber holding CO2 or they can be exposed to a gradually increasing concentration of CO2.
The American Veterinary Medical Association’s 2020 guidelines for carbon dioxide induction suggest that for the humane killing of small rodents, a displacement rate of 30 percent to 70% of the chamber or cage volume per minute is ideal. 5, 31 CO2 concentrations vary by species, based on determined ideal percentages for minimizing distress.
 Frequently Asked Questions:
Frequently Asked Questions:
Some of the questions that usually people ask about this keyword are given below:
1: What is the structure of CO2?
One carbon atom and two oxygen atoms make up a carbon dioxide molecule. Between the carbon and oxygen atoms, there are two double bonds. One sigma and one pi bond make up each double bond. The carbon dioxide molecule has two sigma and two pi bonds in total.
2: Are CO2 and O2 molecules?
The molecular structures of CO2 and O2 differ. Two oxygen molecules make up oxygen, while two oxygen molecules coupled to a central carbon molecule make up carbon dioxide.
3: Is CO2 a small molecule?
Due to their small number of atoms and fairly simple structures, air-liquid gases (the most well-known of which are oxygen, nitrogen, hydrogen, and carbon dioxide…) are referred to as small molecules in the scientific world.
4: Is CO2 a polar molecule?
A polar covalent bond is defined as a binding between two atoms with a difference of 0.4 to 1.7 (on the Pauling scale). The net dipole moment of polar molecules is greater than zero. The dipoles in the linear CO2 molecule, on the other hand, cancel each other out, making the CO2 molecule non-polar.
5: What is the molecular mass of CO2?
Carbon dioxide has a molecular mass of 44.01amu. The mass in grams of one mole of any chemical is known as its molar mass. A mole of carbon dioxide molecules weighs 44.01 grams, while a mole of sodium sulfide formula units weighs 78.04 grams. 44.01g/mol and 78.04g/mol, respectively, are the molar masses.
6: Why is O2 a molecule?
A molecule is made up of two or more atoms that may or may not be of the same element. A compound is made up of two or more bound atoms. As a result, O2 is just a molecule because it has more than one atom and all of its atoms are the same.
7: Which has more molecules O2 or CO2?
A molecule of oxygen is made up of two oxygen atoms and weights 2 x 16 = 32. A total of 44 carbon atoms (weight 12) and two oxygen atoms (mass 16 x 2) make up carbon dioxide. Carbon dioxide should therefore be 44/32 = 1.375 times heavier than an equivalent volume of oxygen.
8: Why CO2 molecule is nonpolar explain?
Because the electronegativities of carbon and oxygen differ, electrons are not shared equally between the two atoms. However, because CO2’s geometry is linear, the two bond dipole moments cancel out, leaving no net molecule dipole moment. The molecule is thus non-polar.
9: Is CO2 nonpolar covalent?
Molecules with more than one type of covalently bound nonmetal atom, such as carbon dioxide gas (CO2), remain nonpolar if their atoms have nearly equal pull. Even big molecules such as hexane gasoline (C6H14) are nonpolar and symmetrical.
10: How do you identify co2?
When carbon dioxide combines with calcium hydroxide solution, it forms a white calcium carbonate precipitate. Limewater is a calcium hydroxide solution. Limewater becomes milky or murky white when carbon dioxide is bubbled through it.
Conclusion:
Carbon dioxide is pumped into or close to producing oil wells, usually under supercritical conditions, when it becomes miscible with the oil. It functions as a pressurizing agent and, when dissolved in subsurface crude oil, decreases viscosity and changes surface chemistry. Carbon dioxide, both liquid and solid, is a key refrigerant in the food business.
Related Articles:
https://howtodiscuss.com/t/what-is-a-molecule-in-chemistry/110336?u=awais_nasir
https://howtodiscuss.com/t/molecular-geometry/112314?u=awais_nasir
https://howtodiscuss.com/t/methane-molecule/108476?u=awais_nasir