O3 molecular geometry is bent at 116.8 degrees, making it three-atomic in structure. Oxygen has only one pair of electrons in the O3 molecule. The Lewis structure of the Ozone molecule is essential for understanding its hybridization, polarity, and molecular geometry. Many individuals today wonder why it is vital to know a molecule or compound’s Lewis structure.
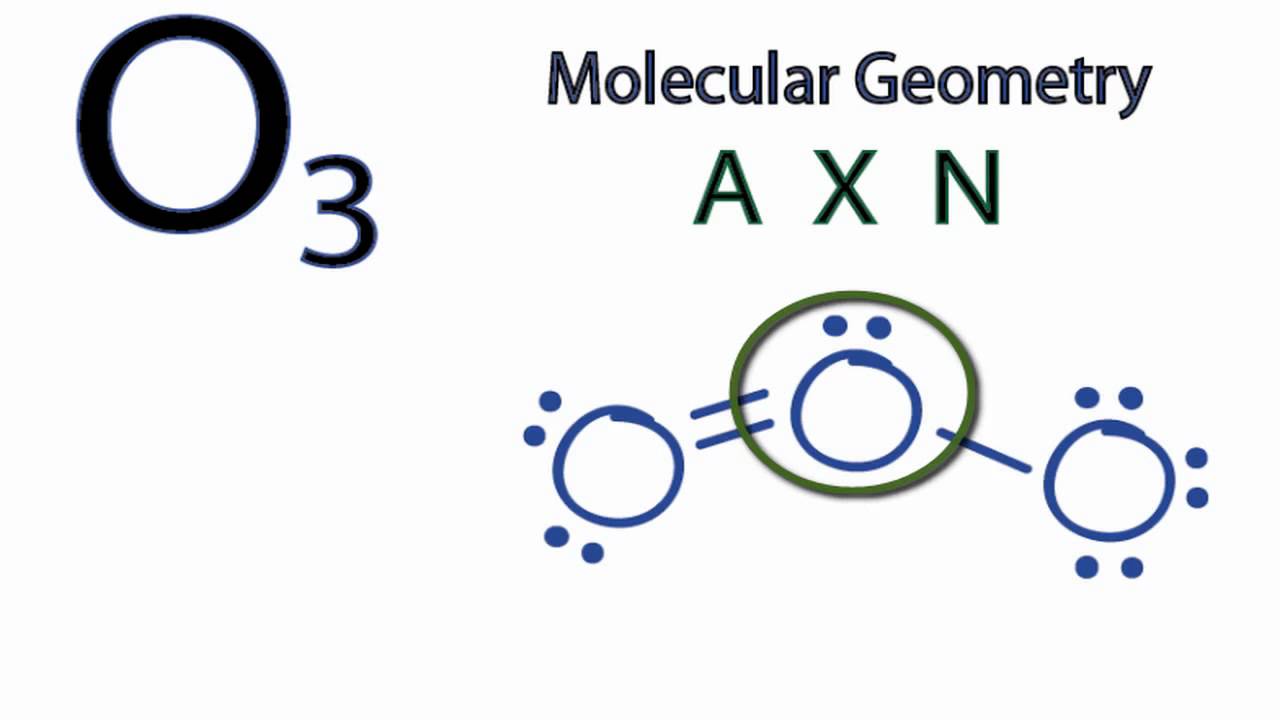
 O3 Molecular Geometry
O3 Molecular Geometry
Ozone is a typical illustrative case study in Lewis structure analysis. Three oxygen atoms make up the molecule of Ozone. In the fundamental chemical equations, it is denoted by the symbol O3.
Understanding the structure of an atom, its electrons, and how they interact with one other is the straightforward answer to this issue. Octet rule provides the foundation of Lewis’s framework. For the molecule to be stable, it must have eight electrons in its outer shell or orbit.
The number of valence electrons in a molecule can be figured out using the Lewis structure. As you may have guessed, valence electrons play a role in the formation and dissolution of bonds. Bonding pairs of electrons are the electrons that participate in bond formation.
In the Lewis structure, these electrons are depicted by drawing lines. Non-bonding pairs of electrons, on the other hand, are those electrons that do not participate in the formation of a bond. These electrons are depicted by a circle of dots surrounding the atom’s core.
 Valence Electrons in O3 (O3V)
Valence Electrons in O3 (O3V)
Every molecule of Oxygen in Ozone or O3 has six valence electrons. The total amount of electrons, in this case, is 18 because there are three oxygen molecules. This means that the Ozone molecule has access to 18 valence electrons.
 The Lewis Structure of O3
The Lewis Structure of O3
Because of the octet rule, the core atom should be the first to have eight electrons in its outer shell in this arrangement. As a result, one Oxygen molecule occupies the centre, while the other two are located on either side.
The eight electrons in the outermost orbit of the centre atom keep the atom stable since it has one lone pair of electrons. A centre atom must make a double bond and a single bond with an oxygen molecule on each side of it to meet the octet rule.
Both Oxygen molecules have a similar electronegativity and structure, which causes a constant movement of the double bond from one molecule to the other.
 The Resonance Structures of O3
The Resonance Structures of O3
There are two double bonds and one single bond between the oxygen molecules that make up the Ozone’s structure, making it unique. Resonant lewis’s structure is the result of these bonds’ constantly changing positions.
Resonance refers to the continual reformation of the links between Ozone’s three atoms. The atom making a single bond will have a charge of -1, while the central atom in the Lewis structure will have +1.
There are two major resonance structures in ozone (O3), each of which contributes equally to the molecule’s hybrid structure. Both configurations provide the 18 valence electrons required - six from three bonds and twelve from the oxygen atoms. 10-Feb-2015
 O3 Hybridisation
O3 Hybridisation
Chemists use the term “hybridization” to refer to the process of creating a new orbital by fusing atoms with varying energies. Knowing a molecule’s Lewis structure makes figuring out its hybridization a cinch.
Oxygen’s outermost shell has eight electrons, hence the atom’s hybridization will be the sp2 configuration. The 2s orbital has two electrons, whereas the 2p orbitals have six electrons each.
The hybridization of the central oxygen atom is sp2 because there are electrons in one s orbital and two p orbitals. Hybridization occurs in the other two oxygen atoms as well.
Sp2 hybridization and sp3 hybridization are both possible since there is a single pair of electrons that causes resonance in the Ozone structure. When it comes to hybridization, we always look at the central atom, therefore in Ozone’s case, sp2 hybridization is the result.

Summary
To create a resonance structure, a single and a double bond between the core oxygen and its flanking oxygens swap locations. There is one formal Positive charge on the central oxygen, while the other oxygens share a negative charge between them. As a result, the dipole moment of ozone is unchanging.
 Molecular Structure of O3
Molecular Structure of O3
Ozone’s molecular geometry can now be determined thanks to the molecule’s hybridization. This suggests it should be trigonally planar because it has sp2 hybridization. However, due to the Ozone’s resonance and lone electron pair structure, the angle here between molecules is a little less than 120 degrees.
 Angles of O3 Bonding
Angles of O3 Bonding
To put it another way, the bonding electrons’ repulsive force is always lower than the repulsive force between bonding electrons. The angle decreases from 120 degrees to 116 degrees since there is only one pair of lone electrons.
 O3 Spherical Shape
O3 Spherical Shape
Trigonal planar shapes are warped when the angle is reduced from 120 degrees to 116 degrees. Because of its distortion, Ozone is frequently described as having a bent or planar shape.
 O3 Polar or Nonpolar
O3 Polar or Nonpolar
Each molecule’s polarity is determined by the molecular geometry. The valence electrons of this Ozone molecule have bent it in this position. Due to their sp2 hybridization, all three Oxygen molecules are not linear.
This molecule has a net dipole because the molecules’ dipole interactions are not neutralized by their non-linear structure. As a result, Ozone can be described as polar due to its polarity. A single pair of electrons on the Ozone’s centre is responsible for its polarity.
 O3’s Lewis Structure
O3’s Lewis Structure
O3 is the molecular formula for ozone, which we’ll be dealing with here. Since O3 has a Lewis structure, our focus here will be on discovering it. Three oxygen atoms make up the ozone. In the periodic table, Oxygen is in group VI with an atomic number of 8. In this way, it contains six valence electrons.
Ozone has a total of 3*6 valence electrons. As in triiodide ion, here, all of the atoms are oxygen, just like in triiodide. This means that one of the three atoms will take centre stage, and the other two will be placed on each side of it.
Step 3 will be used to sketch the skeletal structure of ozone. To complete the octet, place the 18 valence shell electrons (total count) while sketching. According to our knowledge of the periodic table, we can position six electrons around each oxygen, as previously mentioned.
The side oxygen atoms have both reached octet, as can be seen in the image. Each of them is encircled by eight electrons. Only six electrons surround the centre atom.
This is what we must do to comply with the octet rule:
One of the lateral oxygen atom’s electrons must be transferred to the central atom by shifting two electrons from that atom. We’ve now achieved success with the octet creation method. Regarding the central O atom, we now have a double connection and one single bond.
We nowadays have resonance structures because we might have drawn in either direction. The final Sample Containing or electron dot architecture of O3 has been completed after confirming the formal charge.

 Ozone and the Significance of its Presence
Ozone and the Significance of its Presence
Three oxygen atoms combine to form ozone (O3), a highly reactive gas. Atmospheric carbon dioxide is both a natural and man-made substance. mid-to low-level stratosphere (the troposphere). Ozone can either benefit or harm life on Earth, depending on its location in the atmosphere.
Ozone forms naturally in the stratosphere when sunlight’s ultraviolet (UV) energy interacts with oxygen molecules (O2). Protecting Earth’s surface against damaging UV radiation is a primary function of the “ozone layer,” which extends six to 30 miles above the surface.
Photochemical interactions between volatile organic compounds (VOCs) and nitrogen oxides (NOx) are the primary sources of ground-level ozone, which we breathe (NOx).
Summertime temperatures and sunlight have historically been recognised as a primary factor in the formation of these reactions, which lead to greater levels of ambient ozone concentrations.
Some high elevation places in the Western United States with substantial VOC and NOx emissions have created ozone while snow is on the ground and temperatures are near or below freezing throughout the past decade.
Smog and haze, both of which are commonly associated with ozone, can occur throughout the year in some southern and mountainous regions but are more common in the summer.
There is naturally occurring VOC and NOx, but the vast majority of ground-level ozone is produced by interactions between man-made VOC and NOx. Chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops are major contributors to VOC emissions.
High-temperature combustion is the primary source of nitrogen oxides. Power plants, industrial furnaces and boilers, and automobiles all contribute significantly.
To put it another way: Ozone gas, an inorganic compound with the chemical formula O3, is found in the upper layers of the Earth’s atmosphere. Ozone is a colourless gas with a strong odour (similar to chlorine).
Although this layer has a high concentration, it is still relatively low in contrast to other stratospheric gases. Most of the sun’s UV light is absorbed by the Ozone layer. Over the poles, the ozone layer is thicker than at the equator.
The ozone layer shields the planet from the sun’s harmful ultraviolet (UV) rays.
Life on Earth would be extremely difficult without the protective ozone layer that exists above our heads. Plants and plankton, the primary source of food for much of the ocean’s life, are unable to thrive in high levels of ultraviolet radiation.
Skin cancer, cataracts, and weakened immune systems might all result from a decrease in the Ozone Layer. Protecting the planet from the Sun’s harmful ultraviolet (UV) rays is one of the primary functions of ozone.
Life on Earth would be extremely difficult without the protective ozone layer that exists above our heads. Plants and the planktons that feed the majority of ocean life cannot thrive in conditions of high UV exposure.
Skin cancer, cataracts, and a weakened immune system would all be made more common if the Ozone Layer were to be breached. Ozone levels have been disrupted since the 1970s by human activities.
CFCs, a class of chlorine-containing compounds, have contributed to the thinning of the Earth’s ozone layer. Human activity releases chlorine and bromine-containing chemicals into the atmosphere.
When particular meteorological conditions are combined with certain chemicals, processes in the Ozone Layer occur, destroying ozone molecules.
Even though the ozone layer is being depleted all around the world, it is particularly severe in the Antarctic and is known as the “Ozone Hole.” Depletion in the Arctic is also increasing recently.
Summary
The centre O atom in O3 forms a double bond and a coordinate bond with the other O atom by giving an electron pair. On the centre O, there is also a single pair. One orbital is occupied by each of the triple bonds, i.e., sp3 hybridised.
 Frequently Asked Questions - FAQs
Frequently Asked Questions - FAQs
Following are the most Frequently Asked Questions.
 What’s O3’s molecular structure like?
What’s O3’s molecular structure like?
O3 has bent molecular geometry. In the trigonal planar electron geometry, ozone has three electron groups surrounding the centre oxygen. Weirdness in the molecular structure:
 What gives O3 its curved form?
What gives O3 its curved form?
Because of valence shell electron pair repulsion (VSEPR), the electron cloud of the two oxygen atoms will be repelled by electrons. O3 molecules will have a bent molecular geometry or V-shape if the end O groups are pulled down.
 Do you know why there is so much variation in ozone angle than in water?
Do you know why there is so much variation in ozone angle than in water?
The only difference is that water has two lone pairs of electrons surrounding the core oxygen atom, which is exactly what happens in the water molecule. As a result of this, the bond angle of the ozone molecule is larger compared to the water molecule; this means that it is less twisted.
 O3 isn’t a triangle, so what gives?
O3 isn’t a triangle, so what gives?
Steric hindrance prevents ozone from forming a triangle structure with each O atom forming the expected two bonds, although it possesses three oxygen atoms. Oxygen, on the other hand, only makes one link, dispersing any remaining negative charge across the molecule.
 Is there a resonant frequency to O3?
Is there a resonant frequency to O3?
Ozone, or O3, has two primary resonance structures that contribute equally to the overall hybrid structure of the molecule. There are 18 valence electrons required for each structure: six from three bonds and twelve from the oxygen atoms.
 Do you know O3 is a polar or nonpolar molecule?
Do you know O3 is a polar or nonpolar molecule?
Ozone is a polar molecule. A non-bonding pair of electrons on the central atom makes it possible for ozone to have two resonance forms, both of which have two double bonds and so result in a “bent” molecular geometry. Because of the bent geometry, ozone has an overall dipole moment and is polar because of this.
 When compared to oxygen, why is ozone more reactive?
When compared to oxygen, why is ozone more reactive?
As a general rule, ozone is less stable than oxygen. Oxygen and free radicals of the element known as nascent oxygen rapidly dissociate and take part in reactions, making ozone more reactive than pure oxygen.
 What’s the reason why O3 is not cyclic?
What’s the reason why O3 is not cyclic?
Bond length 127.8 pm in the ozone structure lies between a single bond (148 pm) and a double bond (256 pm) (bond length 110 pm). It is not cyclic and is a resonance hybrid of two structures. sp3 hybridised oxygen atoms are present in the structure. It has a curved form.
 Is there a chance that O3 exists?
Is there a chance that O3 exists?
Three atoms of oxygen make up the molecule known as ozone (O3). In contrast to its beneficial role in shielding organisms from the sun’s harmful UV rays, tropospheric ozone is considered a contaminant by many scientists. Ozone is a triatomic molecule because it has three oxygen atoms in each of its three parts.
 Why is there a second bond in O3?
Why is there a second bond in O3?
The ozone molecule has a single link between two oxygen atoms, as well as a single bond and a coordinate bond (which together form the double bond). Because there is no resonance effect as in a normal arrangement, single bonds between all of the oxygen atoms will be extremely unstable.
Conclusion
The stratosphere, at altitudes ranging from 9 to 18 miles (15 to 30 kilometres), contains the majority of Earth’s ozone (see the figure below). ozone has three oxygen atoms in each of its atoms. The stratosphere is continually producing and destroying ozone molecules. Ozone forms naturally in the stratosphere when sunlight’s ultraviolet (UV) energy interacts with oxygen molecules (O2).
Protecting Earth’s surface against damaging UV radiation is a primary function of the “ozone layer,” which extends six to 30 miles above the surface. Because all of the ozone’s electrons are coupled, it is diamagnetic. O2, on the other hand, is paramagnetic due to the presence of two unpaired electrons. Ozone, or O3, is a colourless, odourless gas that is extremely unstable and toxic.
Related Articles
1- O3 Molecular Geometry
2- Is O3 polar or non polar?
3- O3 Molecule