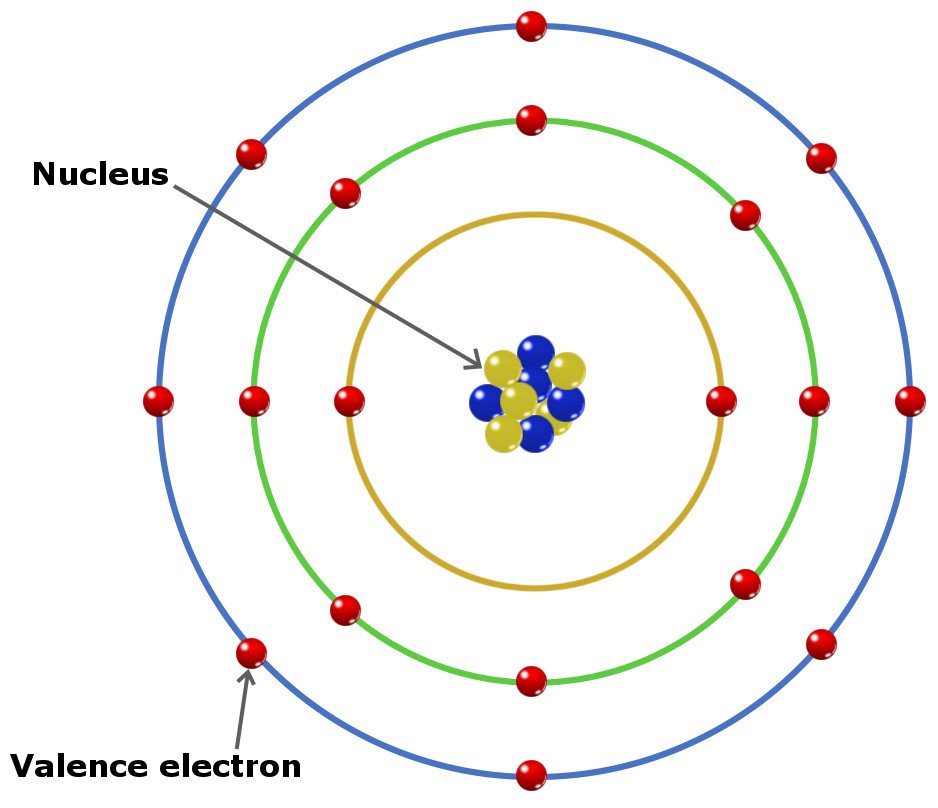
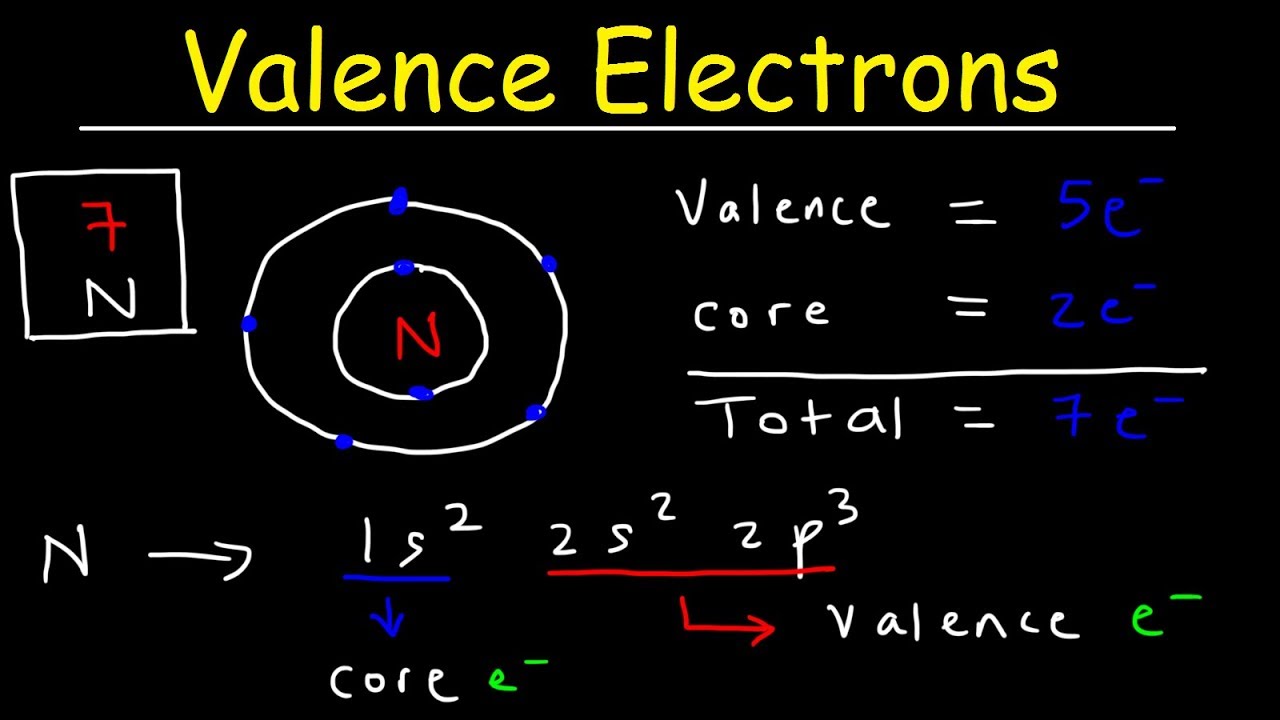
Valence electron, In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom.And that can participate in the formation of a chemical bond if the outer shell is not closed; in a single covalent bond, both atoms in the bond contribute one valence electron to form a shared pair.
What is a valence electron?
What is a valence electron? the presence of valence electrons can decide the component’s substance properties, like its valence regardless of whether it might bond with different components and, assuming this is the case, how promptly and with the number of.
-
Along these lines, a given component’s reactivity is exceptionally subject to its electronic setup. For a fundamental gathering component, a valence electron can exist just in the peripheral electron shell; for a changing metal, a valence electron can likewise be in an inward shell.
-
An iota with a shut shell of valence electrons (relating to a respectable gas setup) will, in general, be synthetically dormant.
-
Particles with a couple of valence electrons above a shut shell are profoundly receptive due to the moderately low energy to eliminate the additional valence electrons to shape a positive particle.
-
A molecule with a couple of electrons under a shut shell is receptive because of its inclination either to acquire the missing valence electrons and structure a negative particle, or, more than likely to share valence electrons and structure a covalent bond.
-
Like a center electron, a valence electron can assimilate or deliver energy as a photon. An energy gain can trigger the electron to move (leap) to an external shell; this is known as nuclear excitation.
-
Or then again the electron can even break liberated from its related particle’s shell; this is ionization to frame a positive particle. At the point when an electron loses energy (in this manner making a photon be transmitted), then, at that point, it can move to an inward shell which isn’t completely involved.
Meaning of valence electron
An electron of a particle, situated in the peripheral shell (valence shell ) of the iota, that can be moved to or imparted to another molecule.
-
Valence electron, any of the key adversely charged particles in the peripheral district of iotas that goes into the arrangement of synthetic bonds. Whatever the sort of compound bond (ionic, covalent, metallic) between molecules, changes in the nuclear construction are limited to the peripheral, or valence, electrons.
-
They are all the more feebly drawn to the positive nuclear core than are the inward electrons and accordingly can be shared or moved during the time spent holding with nearby iotas. Valence electrons are additionally associated with the conduction of electric flow in metals and semiconductors.
Electron’s meaning could be a little clearer
A valence electron is an electron in an external shell of a particle that can take part in framing compound bonds with different iotas. In a solitary covalent bond, the two molecules in the bond contribute one valence electron to frame a common pair.
-
The presence of valence electrons can decide the component’s substance properties and regardless of whether it might bond with different components.
-
For a primary gathering component, a valence electron must be in the furthest electron shell. Be that as it may, in a changing metal, a valence electron can likewise be in an inward shell.
-
A valence electron is an electron that is probably going to be associated with a synthetic response. They are ordinarily the electrons with the most noteworthy worth of the central quantum number, n.
-
One more method for considering valence electrons is that they are the peripheral electrons in a particle, so they are the most helpless to cooperate in synthetic bond development or ionization.
-
The least difficult method for recognizing the valence electrons is to search for the biggest number in the electron design of a molecule (the foremost quantum number).
-
It’s quite significant the IUPAC meaning of valence is for the single most elevated valence esteem that is shown by a molecule of a component.
-
Be that as it may, in commonsense use, fundamental gathering components of the intermittent table might show any valence from 1 to 7 (since 8 is a finished octet).
-
Most components have favored upsides of valence electrons. The antacid metals, for instance, quite often show a valence of 1. The antacid piles of the earth will quite often show a valence of 2.
-
The incandescent lamp ordinarily has a valence of 1, yet may now and again show a valence of 7. The progress metals might show a scope of valence esteems because the most noteworthy energy electron subshell is just to some extent filled. Those iotas become more steady by discharging the shell, half-filling it, or filling it.
Models
Magnesium’s ground state electron arrangement is 1s22s2p63s2, the valence electrons would be the 3s electrons since 3 is the most elevated head quantum number.
Bromine’s ground state electron setup is 1s22s2p63s2p6d104s2p5, the valence electrons would be the 4s and 4p electrons.
Summary
Valence electron is an electron in the external shell related with an iota, and that can take an interest in the arrangement of a synthetic bond assuming the external shell isn’t shut; in a solitary covalent bond, the two iotas in the bond contribute one valence electron to frame a common pair.
Valency and Valence Electrons
Definition, and Connection
Component iotas have an innate ability to bond with other component molecules. A particle is shaped when at least two molecules of the equivalent or various components join to frame a steady design.
-
A particle of sodium chloride is shaped when an iota of sodium and a particle of chlorine join together, while a particle of hydrogen fluoride is framed when a particle of hydrogen and an atom of fluorine consolidate.
-
Every particle has some capacity for blending in with different iotas. Valency alludes to a component’s capacity to join its constituent iotas. Hydrogen and chlorine are accepted to have a similar valency. The valency of different components is dictated by contrasting them with hydrogen or chlorine.
Models
Molecules are comprised of electrons, protons, and neutrons. For the most part, protons and neutrons lie at the focal point of an iota called the core and electrons rotate around the core in a decent way called circles or shells.
-
The shells are named ‘K’, ‘L’, ‘M’, 'N, etc. The deepest shell is the ‘K’ shell which contains up to 2 electrons, trailed by the ‘L’ shell which contains up to 8 electrons followed by the ‘M’ shell which contains up to 18 electrons and afterward the ‘N’ shell which contains up to 32 electrons. The various kinds of molecules meet up and structure components.
-
Construction of fraction.
-
Construction of Iota.
What is Valence electrons?
The electrons in the furthest shell around a nuclear core are known as valence electrons. Valence electrons are significant in light of the fact that they give itemized data on a component’s substance attributes,
For example, regardless of whether it is electronegative or electropositive in nature, or the bond request of a synthetic compound, which demonstrates the number of securities that might be shaped between two molecules.
Hence, valence electrons are characterized as,
-
The number of electrons present in the fringe shell of a part that participates in substance holding are called valence electrons.
-
For example, Lithium (Li) has an atomic number identical to 3. Its electronic plan is K = 2 and L = 1, this induces, K subshell to be totally filled while L subshell, for instance, the uttermost shell has only 1 electron.
-
Changes in the atomic plan are limited to the electrons in the uttermost shell, for instance, valence electrons, paying little brain to the sort of substance correspondence between particles, whether or not it an ionic, covalent, or metallic security.
-
A valence electron is an electron that is added to a molecule and may be used to develop an engineered correspondence; in alone covalent security, the two particles contribute one valence electron to outline a typical pair. The number of valence electrons can impact the compound ascribes of a part similarly to its ability to team up with various parts:
-
A valence electron in a primary gathering component must be in the peripheral electron shell.
-
Artificially, a particle with a shut shell of valence electrons is inactive. Since the extra valence electrons are immediately taken out to make a positive particle, an iota with a couple of valence electrons in excess of a shut shell is amazingly responsive.
-
Due to a propensity to either get the missing valence electrons (making a negative particle) or offer valence electrons, a molecule with a couple of valence electrons less than a shut shell is additionally incredibly responsive (along these lines shaping a covalent bond).
-
A valence electron, similar to an electron in an inward shell, may get or deliver energy as a photon. Nuclear excitation happens when an electron acquires sufficient energy to relocate (leap) to an external shell.
-
Ionization happens when an electron splits from its related molecule’s valence shell, bringing about the development of a positive particle. At the point when an electron loses energy (thus radiates a photon), it may move to an inward shell that isn’t totally involved.
Assurance of Valence Electrons in a Molecule
Albeit the quantity of shells ascends as we travel down a gathering, the quantity of valence electrons stays steady.
While the quantity of valence electrons increments by one after some time, the quantity of shells stays steady. The quantity of shells around a component’s core is shown by the period number (column number).
Valence electrons and open valence’s
A valence electron is an electron that is related to a molecule, and that can partake in the arrangement of a synthetic bond; in a solitary covalent bond, the two iotas in the bond contribute one valence electron to frame a common pair.
-
The presence of valence electrons can decide the component’s compound properties and regardless of whether it might bond with different components: For a fundamental gathering component, a valence electron must be in the furthest electron shell.
-
An iota with a shut shell of valence electrons (relating to an electron arrangement s2p6 ) will in general be artificially latent. A molecule with a couple of valence electrons in excess of a shut shell is profoundly responsive, on the grounds that the additional valence electrons are effortlessly eliminated to shape a positive particle.
-
An iota with a couple of valence electrons less than a shut shell is likewise profoundly responsive, as a result of a propensity either to acquire the missing valence electrons (along these lines framing a negative particle) or to share valence electrons (in this manner shaping a covalent bond).
-
Like an electron in an internal shell, a valence electron can ingest or deliver energy as a photon. An energy gain can trigger an electron to move (leap) to an external shell; this is known as nuclear excitation.
-
Or on the other hand the electron can even break liberated from its related particle’s valence shell; this is ionization to shape a positive particle. At the point when an electron loses energy (in this way making a photon be discharged), then, at that point, it can move to an inward shell that isn’t completely involved.
The number of valence electrons
The number of valence electrons of a component can be controlled by the intermittent table gathering (vertical section) in which the component is ordered.
Except for bunches 3–12 (the change metals), the units digit of the gathering number recognizes the number of valence electrons that are related with a nonpartisan particle of a component recorded under that specific segment.
Here, I present a flow chart of elements groups:
|Group 1 (I)|Alkali metals|
| — | — |
|Group 2 (II)|Alkaline earth metals|
|Groups 3-12|Transition metals|
|Group 13 (III)|Boron group|
|Group 14 (IV)|Carbon group|
|Group 15 (V)|Pnictogens|
|Group 16 (VI)|Chalcogens|
|Group 17 (VII)|Halogens|
|Group 18 (VIII or 0)|Noble gases|
The overall strategy for counting valence electrons is for the most part not helpful for progress metals. Rather the adjusted d electron count strategy is utilized. With the exception of helium, which has just two valence electrons.
The Idea of Open (“Valence”)
The valence (or valency) of a component is a proportion of its consolidating power with different particles when it structures synthetic mixtures or atoms. The idea of valence was created in the last 50% of the nineteenth century and was fruitful in clarifying the atomic design of numerous natural mixtures.
-
The mission for the fundamental reasons for valence lead to the cutting edge hypotheses of synthetic holding, including Lewis structures (1916), valence security hypothesis (1927), sub-atomic orbitals (1928), valence shell electron pair shock hypothesis (1958), and every one of the high-level strategies for quantum science.
-
The consolidating power or proclivity of an iota of a component was controlled by the number of hydrogen particles that it joined with. In methane, carbon has a valence of 4; in smelling salts, nitrogen has a valence of 3; in water, oxygen has a valence of two; and in hydrogen chloride, chlorine has a valence of 1.
-
Chlorine, as it has a valence of one, can be filled in for hydrogen, so phosphorus has a valence of 5 in phosphorus pentachloride, PCl5.
-
Valence graphs of a compound address the network of the components, lines between two components, now and then called securities, addressed a soaked valency for every component.
-
Valence just portrays network, it doesn’t depict the math of atomic mixtures, for sure are currently known to be ionic mixtures or monster covalent designs. The line between iotas doesn’t address a couple of electrons as it does in Lewis charts.
What is Valency?
The valency of a molecule is equivalent to the number of valence electrons that this particle can acquire or lose during synthetic responses. Or then again all in all:
![]() How much hydrogen molecules, chlorine particles, or twofold the quantity of oxygen iotas that one iota of a component might join with is alluded to as its valency.
How much hydrogen molecules, chlorine particles, or twofold the quantity of oxygen iotas that one iota of a component might join with is alluded to as its valency.
![]() The valency of a component alludes to the number of valence electrons in that component that takes part in substance processes.
The valency of a component alludes to the number of valence electrons in that component that takes part in substance processes.
![]() A substance compound is made when at least two parts are consolidated in a specific mass extent. A steady compound is framed when one iota of one component joins with a particular number of particles of another component. All parts have various abilities for blending.
A substance compound is made when at least two parts are consolidated in a specific mass extent. A steady compound is framed when one iota of one component joins with a particular number of particles of another component. All parts have various abilities for blending.
Valency of Metals
A metal is a component with one, two, or three electrons in its valence shell (aside from hydrogen and helium, which are non-metals). In compound cycles, metals will quite often shed their valence electrons and complete their octet. In this manner,
-
Valency of metallic component = number of electrons in its valence shell.
-
Components from Gathering 1, 2, and 13 of the intermittent table have valencies 1, 2, 3 separately which is like the electron arrangement in their last shell.
For Instance:
Sodium (Na)
The Electronic design of Na is 11 (K = 2; L = 8; M = 1). They have 1 electron in their furthest shell. Along these lines, the valency of Sodium is 1.
Magnesium (Mg)
The Electronic design of Mg is 12 (K = 2; L = 8; M = 2). They have 2 electrons in their furthest shell. Along these lines, the valency of Magnesium is 2.
Aluminum (Al)
The Electronic design of Al is 13 (K = 2; L = 8; M= 3). They have 3 electrons in their furthest shell. Along these lines, the valency of Aluminum is 3 as displayed underneath:
Valence electrons of the aluminum
Valency of Non-Metals
Assuming that a component’s valence shell has 5 or 6 or 7 or 8 electrons, it is named a non-metal. To finish its octet, a non-metal requires 3 or 2 or 1 or 0 electrons. Accordingly, a non-metallic component’s valency = 8 – number of electrons in its furthest shell.
Components from Gatherings 15, 16 and 17 of the intermittent table have 5, 6, 7 electrons individually in their last shell so the valency of non-metals is chosen by observing the number of electrons needed to finish its octet design.
For instance:
Nitrogen (N)
The Electronic setup of N is 7 (K = 2; L = 5; M= 0). They have 5 electrons in their peripheral shell. In this way, the valency of Nitrogen is: 8 – 5 = 3
Valence electrons of nitrogen
Oxygen (O)
The Electronic design of O is 8 (K = 2; L = 6; M= 0), They have 6 electrons in their furthest shell. Along these lines, the valency of Oxygen is : 8 – 6 = 2 as displayed underneath:
valence electrons of oxygen
Fluorine (F)
The Electronic design of F is 9 (K = 2; L = 7; M= 0). They have 7 electrons in their furthest shell. Along these lines, the valency of Fluorine is: 8 – 7 = 1.
Valency of Nobel gases
Bunch 18 components of the intermittent table are called respectable gases since they don’t respond by any means as they have the most steady electronic design due to having the greatest number of valence electrons in its external shell. The valency is zero as each honorable gas’ furthest shell is filled thus they don’t lose or acquire any electron.
For Example:
Neon (Ne)
The electronic arrangement of Ne is 10 (K = 2; L = 8; M= 0). They have 8 electrons in their furthest shell. Thus, the valency of Neon is : 8 – 8 = 0.
Connection Among Valency and Valence Electrons
Valency clarifies the bond development of molecules. Though, valence electrons are identified with basic characters. Valency is just an idea or thought and doesnot include transmission of electrons. Though, valence electron includes transmission of electrons in the development of bonds. Both valency and valence electrons are applied for any compound component.
-
Since, just the valence electrons (furthest electrons) take part in substance holding, a component’s valency is dictated by the quantity of valence electrons (peripheral electrons) in its molecule.
-
How much valence electrons in a component’s particle or the quantity of electrons important to finish eight electrons in the valence shell decides the component’s valency. Sodium, for instance, contains one valence electron and consequently a valency of one.
-
Accordingly, the valency of sodium is equivalent to how much valence electrons in its molecule. A metal component’s valency is equivalent to the quantity of valence electrons in its particle overall.
-
Valency of a metal = Number of valence electrons in its particle
-
A valence electron, similar to an electron in an internal shell, may get or deliver energy as a photon. Nuclear excitation happens when an electron acquires sufficient energy to move (leap) to an external shell.
-
Ionization happens when an electron splits from its related iota’s valence shell, bringing about the development of a positive particle. At the point when an electron loses energy (thus produces a photon), it may relocate to an internal shell that isn’t totally involved.
-
Valency of a non-metal = 8 – Number of valence electrons in its molecule
-
The valency of hydrogen is the one special case for this standard. Hydrogen’s valency is equivalent to the quantity of valence electrons, which is one (however hydrogen is a non-metal component).
Corrosionpedia Clarifies Valence Electron
Valence electrons are the electrons in the furthest electron shell of a particle. The quantity of electrons in a molecule’s furthest valence shell oversees its holding conduct.
-
For that reason components whose particles have similar number of valence electrons are assembled in the Occasional Table. Inside each gathering of metals, reactivity increments while moving lower on the table. Inside each gathering of nonmetals, reactivity diminishes with lower positions on the table.
-
Components are most steady when they have every one of the eight valence electrons. This is known as the octet rule.
-
The main components that have this electron design are the respectable gases, subsequently they are dormant. Any remaining components gain, lose or share electrons to accomplish a full arrangement of valence electrons.
-
Non-metals have a solid draw on their valence electrons, so they will more often than not gain electrons to acquire soundness and become more like a respectable gas. Since metals have free valence electrons, they generally lose electrons to acquire security.
At the point when a metal loses electrons, it has a bigger number of protons than electrons and is accordingly a positive particle.
-
Valence electrons are likewise answerable for the electrical conductivity of a component; subsequently, a component might be named a metal, nonmetal or semiconductor (metalloid). A metal is a component with high electrical conductivity or pliability when in the strong state.
-
A nonmetallic component has low electrical conductivity; it goes about as a cover. A semiconductor has an electrical conductivity that is transitional between that of a metal and that of a nonmetal.
What Are Valence Electronic?
What makes cool compound reactions work? Review those lovely examinations like making a well of magma from baking pop and vinegar or a rocket from Mentos and pop? We wouldn’t have these reactions without valence electrons.
-
Valence electrons are the electrons arranged at the farthest shell of a molecule. Why are these electrons interesting? Since when two atoms interface, the electrons in the fringe shells are the underlying ones to come into contact with each other and are the ones that conclude how a molecule will react in a manufactured reaction.
-
We should imagine a reasonable food, go through restaurant. We go through the way in our vehicle, show up at our hands out the window, and the laborer associates and gives us the food.
-
The whole participation between the vehicle and the restaurant lays essentially on the arms of the laborer and driver at their windows. The arms of the driver and the arms of the laborer are like valence electrons.
-
We should look at specific models base to think about valence electrons.
Diagrams of Oxygen and Neon Particles
For the oxygen molecule, you can see that the uttermost shell has 6 electrons, so oxygen has 6 valence electrons. Neon’s fringe shell has 8 electrons. Neon thusly has 8 valence electrons.
-
The shells of a molecule can tragically hold a predetermined number electrons. Each shell has a particular proportion of subshells (s, p, d, etc) that have a particular proportion of orbitals. Each orbital can hold 2 electrons. The chief shell has one subshell, s, which has one orbital, so it can hold 2 electrons. Irrefutably the quantity of electrons that each shell can hold is:
-
Shell 1 - has subshell s, which has one orbital. It can thusly hold 2 electrons.
-
Shell 2 - has subshells s and p. p has 3 orbitals, so can hold 6 electrons. Add the two that subshell s can hold, and we understand that shell 2 can hold 8 full scale electrons
-
Shell 3 - has subshells s, p, and d. d has 5 orbitals, so can hold 10 electrons. Shell 3 can hold an amount of 18 electrons.
-
Take magnesium, which has an amount of 12 electrons. Expecting that we draw the electrons for magnesium, you’ll have 3 shells.
-
The essential shell will take its most noteworthy, 2, in this manner will the ensuing shell, with 8. The extra two electrons will include the third, outer, shell. Thusly, magnesium has 2 valence electrons.
-
Phosphorus, on the other hand, has 15 electrons.
-
It will similarly have three shells, and the first and second shells are both totally involved. The third shell will house the extra 5 electrons, which suggests phosphorus has 5 valence electrons.
Electron Arrangement
Before we hop into valence electron configuration, let us first review electron arrangement. An electron arrangement is the arrangement of electrons around the center of a molecule, comparably as we’ve been looking at as yet. Each molecule has its own circumstance on the irregular table, and you can notice the electron plan by knowing where the atom is set on the table.
-
The atomic number of a bit in the ground state is pretty much as old as number of electrons. For example, sodium has an atomic number of 11 and magnesium has an atomic number of 12. Along these lines, sodium should contain 11 electrons in its electron game plan and magnesium should contain 12.
-
Each particle has an orbital in a particular solicitation. We can continue to draw the electrons like we have been doing, yet there is a more restricted and more straightforward technique for doing it. This is known as the spdf documentation.
-
An intermittent table is parceled into s, p, d, and f blocks. We can use the picture to choose the electron arrangement of a molecule. It’s important’s indispensable the quantity of electrons include the subshells s, p, d, and f.
-
Could we get the electron plan for aluminum, which has an atomic number of 13. The two parts are shown on the discontinuous table.
-
From their positions, we can choose the electron plan. Aluminum is quite far in the third line, so its electrons have the first and second segments totally, and the third line somewhat. We count from left to right the entire way to aluminum, recording each one as we go. We can create its electron plan as:
-
1s^2 2s^2 2p^6 3s^2 3p^1
-
In ‘1s^2’ the ‘1s’ implies the essential shell’s subshell s, and the ‘2’ suggests the 2 electrons it will hold. In ‘3p^1’, ‘3p’ implies the third shell’s subshell p, and the ‘1’ means it’s simply holding one electron. Anyway p subshells can hold an amount of 6 electrons, aluminum simply has 13 electrons, with the previous subshells holding the vast majority of them.
The electron arrangement of calcium
Calcium has an atomic number of 20. It’s beyond what many would consider possible on the fourth section. So we think about the same way aluminum, beyond what many would consider possible until we show up at calcium.
Another strategy for making the electron game plan is by using this model and reviewing that it.
What are valence electrons?
-
Valence electrons and the discontinuous table
-
Instructions to find valence electrons
-
Valence electrons of parts other than change parts the major social event parts
Suggested Scrutinizing
Deeply. Valence electrons are of basic importance since they advance significant comprehension into a part’s manufactured properties: whether or not it is electronegative or electropositive in nature, or they show the bond solicitation of a substance compound the amount of bonds that can be molded between two atoms.
Since covalent securities are molded through the sharing of electrons in the last shell, the number exhibits the quantity of protections that may be outlined.
What are valence electrons?
Valence electrons are the electrons that are arranged in the fringe shell of a bit. All things considered, these are the electrons that can be obtained or lost during an engineered reaction.
Where could valence electrons be?
Despite the sort of engineered association between particles, be it an ionic, covalent or metallic bond, changes in the atomic plan are confined to the electrons in the farthest shell, for instance valence electrons.
Directions to Find The Amount Of Valence Electrons
The most basic system is imply the atomic arrangement of a part and basically remember the electrons for the fringe shell. Regardless, this would be an unquestionably challenging endeavor, as we may have to tunnel through course readings to notice plans we don’t have even the remotest clue.
![]() In any case, there’s no convincing excuse to be stressing out, as there’s significantly less complicated method of concluding this longed for number. This is a more summarized approach that simply requires bringing one minimal brilliant rectangular piece of paper a periodic table.
In any case, there’s no convincing excuse to be stressing out, as there’s significantly less complicated method of concluding this longed for number. This is a more summarized approach that simply requires bringing one minimal brilliant rectangular piece of paper a periodic table.
![]() To conclude the amount of valence electrons of a part, we simply need to imply a periodic table and mission for the spot of the part in it.
To conclude the amount of valence electrons of a part, we simply need to imply a periodic table and mission for the spot of the part in it.
Valence electrons and an intermittent table
An intermittent table is an ideal blueprint of all parts viewed as up until this point. The parts are coordinated from left to right in rising solicitation of their atomic numbers or the amount of protons or electrons they contain.
The best strategy to Find The Amount Of Valence Electrons In A Part Science ABC
The parts are apportioned into four classes: essential social occasion parts, change parts, lanthanides and actinides. The last two are also suggested as inward change parts.
The table contains 18 areas out and out, authoritatively known as social affairs, similarly as lines, formally known as periods. There are 7 lines in the subtable above and 2 lines perceiving the more exceptional parts under. The change parts structure an augmentation, or support the advancement between the parts in Social affairs 2 and 13.
How to track down valence electrons
At the point when we go down a gathering, the quantity of valence electrons continues as before, albeit the quantity of shells increments.
![]() While the period number demonstrates the quantity of shells, the gathering number shows the quantity of valence electrons in the peripheral shell. In particular, the number during the ones’ place. Be that as it may, this is just valid for the fundamental gathering components—the components occupying bunches 1-2 and 13-18.
While the period number demonstrates the quantity of shells, the gathering number shows the quantity of valence electrons in the peripheral shell. In particular, the number during the ones’ place. Be that as it may, this is just valid for the fundamental gathering components—the components occupying bunches 1-2 and 13-18.
![]() The standard is irrelevant to the progress and internal change components (we’ll get to that explanation in a moment). For example, Sodium (Na) dwells in Period 3, Gathering 1, which suggests that it has 3 shells and a solitary electron in its valence shell.
The standard is irrelevant to the progress and internal change components (we’ll get to that explanation in a moment). For example, Sodium (Na) dwells in Period 3, Gathering 1, which suggests that it has 3 shells and a solitary electron in its valence shell.
![]() Or on the other hand, you can think about chlorine in Gathering 17. In like manner, to decide its valence electrons, we should just look for the number in its ones’ place: 7. True to form, that is by and large the quantity of electrons in its valence shell.
Or on the other hand, you can think about chlorine in Gathering 17. In like manner, to decide its valence electrons, we should just look for the number in its ones’ place: 7. True to form, that is by and large the quantity of electrons in its valence shell.
Instructions to Track down The Quantity Of Valence Electrons In A Component Science ABC
This strategy for essentially alluding to the occasional table and deciding the comparing bunch number has disposed of the problem and intricacy that once went with the difficult quest for individual nuclear designs.
-
Shouldn’t something be said about the valence electrons of the components in the middle? Obviously we should not fail to remember the lanthanides and actinide.
-
A speedy clarification of how shells are loaded up with electrons
-
Change components are very little not quite the same as metals that go shoulder to ought to
How To Find The Number Of Valence Electrons In An Element?
The electrons just must be filled from left to right in precisely this request.
If we somehow happened to disseminate electrons unknowingly concerning how the usb-shells are arranged, as displayed in the figure above, Calcium (Ca) with nuclear number 20 would have the design 2,8,10 (2, 2+6, 2+6+2). Any secondary school science course reading will let you know that this isn’t right, as the exact setup is 2,8,8,2.
-
However, on the grounds that we should maintain the standard, we see that 4s should be filled before 3d, to such an extent that there are presently 8 in the third shell and 2 in the fourth making the setup: 2,8,8,2. Presto! As Richard Feynman would happily shout: The joy of tracking down things out! Unfortunately, the delight is just half-lived the justification for the actual standard, this evident ridiculousness, is past the extent of this article.
-
Alright, since we realize how shells are filled, we can go further to track down the quantity of valence electrons in the change components
Valence electrons of lanthanides and actinides (progress and inward change components)
Think about Scandium (Sc) with its nuclear number of 21. Filling the electrons as per our standard, we see that the 21st electron involves the 3d sub-shell. In any case, as the recently filled fourth shell (4s) has 2 electrons and is clearly the peripheral shell, the quantity of valence electrons is 2.
Additionally, every change component in the fourth time frame should have 2 valence electrons. The explanation being that despite the fact that 3d stretches out beyond 4s, the two electrons arranged in the fourth shell are the occupants of the peripheral shell and legitimately merit the assignment of valence electrons.
How To Track down The Quantity Of Valence Electrons In A Component
Various components among the progress components show this peculiarity, which is likewise seen on the inward change components, because of the equivalent energy levels of f, d and s shells.
-
In synopsis, it can along these lines be said that the quantity of valence electrons for change components and inward progress components shifts in an erratic manner.
-
Albeit the quantity of valence electrons for the progress components can in any case be anticipated – and a large portion of them end up at 2 – such an expectation for the internal change components can’t be imitated.
-
The fanciful conduct of their valence electrons, unendingly shaking and bouncing in hesitation, denies any endeavor to acquire a remarkable stable arrangement making anticipating the quantity of valence electrons exceptionally difficult.
Summary
Valence alludes to the capacity of a molecule or a gathering of compound reinforced iotas to frame synthetic structure with different particles or gatherings of molecules. The valency of a component is controlled by the quantity of external shell (valence) electron.
Frequently Ask Questions
Here, a few inquiries emerge identified with this article who are as per the following:
1.What is valence electron with model?
Valence electrons are the electrons in the furthest shell, or energy level, of an iota. For instance, oxygen has six valence electrons, two during the 2s subshell and four in the 2p subshell. We can compose the design of oxygen’s valence electrons as 2s²2p⁴.
2. How do you have at least some idea what a valence electron is?
For nonpartisan iotas, the quantity of valence electrons is equivalent to the molecule’s primary gathering number. The principle bunch number for a component can be found from its segment on the intermittent table. For instance, carbon is in bunch 4 and has 4 valence electrons. Oxygen is in bunch 6 and has 6 valence electrons.
3. How would you work out valence electron focus?
To ascertain the valence-electron commitment of a component one needs to take away 8 from the all out number of external shell electrons er which is equivalent to the gathering number of the progress component in the intermittent framework. For instance Pd with er= 10 gives just 2 valence electrons
4. How would you track down the electrons?
To work out the quantities of subatomic particles in a molecule, utilize its nuclear number and mass number: number of protons = nuclear number. number of electrons = nuclear number
5. What number of valence electrons does chlorine?
The nuclear number of chlorine is 17. Consequently it has got 7 electrons in its peripheral shell. There are 7 valence electrons in the chlorine particle.
6. What family has 8 valence electrons?
Respectable Gases The Gathering 18 components are the honorable gases. Molecules of the honorable gases have 8 valence electrons, with the exception of helium, which has 2. Particles with 8 valence electrons (or 2, on account of helium) are steady. They are probably not going to acquire or lose electrons or to impart electrons to different particles.
7. What is the rule for finding out how many valence electrons elements in Groups 13 18 have?
Components in Gathering 1 have one valence electron, and components in Gathering 2 have two valence electrons. For Gatherings 13 through 18, the quantity of valence electrons is the gathering number short 10. The exemption for this standard is helium (He).
8. What is the highest valence electron?
On an important level, beyond what 8 electrons can be in the most elevated energy orbitals for holding, however, probably, 9 valence electrons have up until this point been utilized, to be specific in iridium, while osmium, ruthenium, xenon, and hassium have utilized all things considered 8.
9. What is the valence electron pattern for elements in the same group?
Components in a similar Gathering have a similar number of valence shell electrons. Across a time of the Intermittent Table from left to right the quantity of valence electrons increments
10. What is valence in science?
Valence, additionally spelled valency, in science, the property of a component that decides the quantity of different iotas with which a particle of the component can join.
Conclusion
If anyone not known What is a valence electron ? And also its details. Then, I suggest that you must read this article with carefully because in this article, I fully described all details about valence electron.
Related Articles;
You May Also Like;