Sulfur Valence Electrons are 6 in number. Sulfur’s Valency could be as high as 6. The outermost shell of sulfur needs two electrons to complete. So, to attain stability and complete the outermost shell, sulfur gains two electrons.
What Is The Valency Concept?
If we’re talking about the fundamentals of valency, the electrons gained or lost by an atom to complete the outer shell are the basic concepts. For the entire outmost shell, atom stability is determined by gained or lost electrons.
When the outermost shell of an atom is finished, it is said to be stable. An atom’s stability can be achieved by adding or losing electrons for the entire outer shell. The term for this occurrence is valency.
What Is The Valence Shell Concept?
The valence shell refers to an atom’s outermost electron shell. The valence electrons are in the outermost shell of an atom.
What Is The Meaning Of The Idea Of Valence Electrons?
Valence electrons are electrons that are available or present in an atom’s outer shell.
What Is Sulfur’s Valency?
Two methods are used to determine the valency of sulfur. As an example:
1. The valency of Sulfur is determined by the periodic table.
2. The configuration of electrons determines the valency of sulfur.
1. Sulfur’s Value Is Determined By The Periodic Table.
Because sulfur’s atomic weight is 16, you can check it immediately as an element of group 16. In the periodic table, you have the valency of oxygen, and the valency of sulfur is 6.
2. The Configuration Of Electrons Determines The Sulfur’s Valency.
You are aware that the outermost shell of the sulfur atom has six electrons. Sulfur’s valency could be as high as 6. The outermost shell of sulfur needs two electrons to complete. So, to attain stability and complete the outermost shell, sulfur gains two electrons. This means that sulfur must gain two electrons. As shown in the sulfur structure.
With the help of electron configuration, you can now grasp everything. So, let’s write sulfur’s electron configuration. The electron configuration of sulfur is 1s2, 2s2, 2p6, 3s2, 3p4, 3s2, 3p4 is the greatest energy level in the electron configuration.
If you include this, 2 + 4 = 6 .
As a result, the valency is 6.
As a result, 2 electrons were obtained for appropriate sulfur stability or a complete octet in the outermost shell. As a result, sulfur has a valency of 2. In the case of SO2 and SO3, the outermost [shell required six electrons to complete.
As a result, the valency of this molecule, for example:
-
Sulfur (S) – 2
-
Sulfur dioxide (SO2) – 6
-
Sulfur trioxide (SO3) – 6
What Are The Fundamental Notions Of Sulfur Valency In (S), Sulfur Dioxide (SO2), And Sulfur Trioxide (SO3)?
1. Sulfur Dioxide’s Valency (SO2)
-
Sketch the structure of sulfur dioxide first.
-
Sulfur atoms are joined by two oxygen atoms. It is linked to the double bond.
-
The valency of sulfur in sulfur dioxide is +4.
-
As a result, sulfur compounds with a valency of 6 have a valency of 6.
2. Sulfur Trioxide’s Valency (SO3)
-
To begin, sketch the structure of sulfur trioxide.
-
If you look at the structure of sulfur trioxide, you’ll notice that it has three oxygen atoms bonded to one sulfur atom.
-
The valency of sulfur in sulfur trioxide is +6.
-
The valency of O3 in sulfur trioxide is -6.
-
As a result, we can say that sulfur trioxide has a valency of 6.
Configuration Of Sulfur Electrons
Sulfur electron configuration – sulfur is a chemical element that is not metallic. The most reactive chemical element is sulfur. It is water-insoluble. The chemical symbol for sulfur is (S), and its atomic number is 16. Sulfur is a poor electrical conductor.
1. S is the symbol for sulfur
2. Insoluble in water in terms of solubility
3. [Ne] 3s2 3p4 Electron Configuration
4. 2.58 Electronegativity
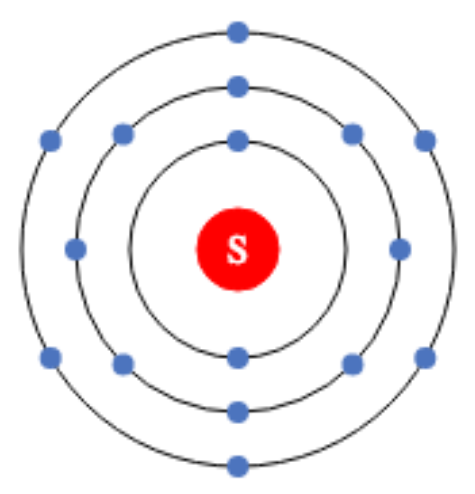
5. 2, 8, 6 electrons per shell
Let’s Look At The Electronic Configuration Of Sulfur.
- Sulfur has an atomic number of 16. Sulfur atoms, on the other hand, have 16 electrons. Let’s look at the subshell of the sulfur atom now .The arrangement of sulfur’s atomic subshells to increase the energy of the fundamental. Only two electrons are placed in the 1s subshell, which has one 1s orbital.
As an example :
- It has a 2s subshell with one 2s orbital where the next two electrons can be inserted.
As an example:
- Shells 2–3 each have a 2p subshell and three 2p orbitals. You have a total of 6 electrons to work with.
As an example:
- Shells 3–4 have a 3s subshell containing 3s orbitals, but only two electrons are placed in 3s orbitals.
As an example:
-
Shell 4 contains a 3p subshell with 3p orbitals, requiring just 4 electrons to complete the sulfur electron configuration. since sulfur has an atomic number of 16 As a result, only four electrons are placed in the 3p orbitals.
-
For the entire electron configuration of sulfur, four electrons are required in the case of 3p orbitals. That is why 4 electrons are placed in 3p orbitals. However, two electrons are unpaired.
If We’re Talking About The Electronic Configuration Of Sulfur’s Group State.
1. The initial two electrons in a sulfur electron configuration go into the first orbitals because the first only has two electrons
2. Sulfur’s next two electrons are placed in 2s orbitals.
3. Sulfur’s next six electrons are now in 2p orbitals.
4. The next two electrons are placed in 3s orbitals.
5. For the sulfur atom, just four electrons are required to complete the electron configuration. Because 16 is the atomic number of sulfur.
6. To complete the electron configuration for the sulfur atom, just four electrons are placed in 3p orbitals. As depicted in the diagram.
7. We can write 1s2 2s2 2p6 3s 3p4 for the sulfur electron configuration, Finley.
Applications Of Sulfur
1. Sulfur is utilized in the manufacture of strong acid. Sulfuric acid, for example.
2. In the chemical industry, sulfur is used.
3. In the textile sector, sulfur is used.
4. In the oil sector, sulfur is used.
5. In the fertilizer industry, sulfur is used.
6. Sulfur is utilized in the making of products such as cement.
Characteristics Of Sulfur
Sulfur is a nonmetallic element that is plentiful and multivalent. Sulfur atoms have the chemical formula S8 and form cyclic octatomic molecules under normal conditions. Sulfur is a brilliant yellow crystalline solid at ambient temperature. Sulfur reacts chemically with every other element except gold, platinum, iridium, tellurium, and noble gases.
The element’s most common commercial application is the manufacturing Of sulfuric acid for sulfate and phosphate fertilizers, as well as other chemical processes. There are three main ways to make sulfur. Sulfur can be mined using wells dug to sulfur deposits using the “Frasch” process.
Neutrons And Protons Of Sulfur
| 1. Name | Sulfur |
|---|---|
| 2. Protons | 16 |
| 3. Neutrons | 16 |
| 4. Electrons | 16 |
| 5. Atomic number | 16 |
Sulfur is a chemical element with the atomic number 16, meaning it has 16 protons in its nucleus. The atomic number, which is represented by the letter Z, is the total number of protons in the nucleus. As a result, the nucleus has a total electrical charge of +Ze, where e equals 1,602 x 10-19 coulombs.
The atom’s neutron number is the total number of neutrons in its nucleus, and it is represented by the letter N. The atomic mass number equals neutron number and the atomic number: N+Z=A. The neutron excess number (D = N – Z = A – 2Z) is the difference between the neutron number and the atomic number.
For stable elements, there are frequently many stable isotopes. Isotopes are nuclides that have the same atomic number and consequently the same element but have different numbers of neutrons. The mass numbers of the usual Sulfur isotopes are 23; 33; 34; 36.
Major Isotopes Of Sulfur
There are 23 isotopes of sulfur, four of which are stable:
1. S-32 (94.99 percent 0.26 percent )
2. S-33 (0.75 percent 0.02 percent )
3. S-34 (4.25 percent 0.24 percent )
4. S-35 (0.01 percent 0.01 percent ).
Number Of Protons, Electrons And Protons In Isotopes Of Sulfur
-
Sulfur-32 consist of 16 protons, 16 neutrons, and 16 electrons.
-
Sulfur-33 contain 16 protons, 17 neutrons, and 16 electrons.
-
Sulfur-34 is made up of 16 protons, 18 neutrons, and 16 electrons.
-
Sulfur-36 is made up of 16 protons, 20 neutrons, and 16 electrons.
During the precipitation of sulfide minerals, isotopic equilibration between solids and liquids can cause minor variations in the S-34 values of co-genetic minerals. Mineral differences can be used to calculate the temperature of equilibration.
The C-13 and S-34 of coexisting carbonate minerals and sulfides can be used to determine the pH and oxygen fugacity of the ore-bearing fluid during ore formation. In most forest ecosystems, sulfate is mostly created by the environment, with some sulfur added by weathering of ore minerals and evaporites.
Sulfur’s isotopic composition has been used to discover pollution sources, and enriched sulfur has been employed in hydrologic research as a tracer. Natural abundance variations can be used in systems with adequate variability in the S-34 of ecosystem components.
The S-34 values of Rocky Mountain lakes expected to be dominated by atmospheric sulfate sources were found to be similar to those of lakes dominated by watershed sulfate sources.
Electrons And Electron Configuration Of Sulfur
The number of electrons in an atom equal to the proton number in the nucleus. As a result, an electron in a neutral Sulfur atom has 16 electrons. Each electron is affected by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
The atomic number distinguishes chemical elements because atoms’ chemical behavior is determined by the number of electrons and their arrangement. The configuration of these electrons is dictated by quantum mechanics laws.
Chemical bonding behavior is determined by the number of electrons in each element’s electron shell, particularly the outermost valence shell. The periodic table lists the elements in ascending atomic number Z-order.
-
Sulfur’s electron configuration is [Ne] 3s2 3p4.
-
There are four potential oxidation states: +4,6/-2, +4,6/-2, and +4,6/-2.
Except for noble gases, sulfur reacts with almost every other element, even the ostensibly inert metal iridium (yielding iridium disulfide). High temperatures are required for several of these reactions. It’s one of the world’s most reactive components.
Summary
Sulfur-32 has 16 electrons, 16 protons, and 16 neutrons. The most prevalent commercial application is the manufacturing of sulfuric acid for sulfate and phosphate fertilizers. There are 23 isotopes of sulfur, four of which are almost stable. The beta decay time of 35S in an electrically neutral atom is 170.3(7) minutes.
The Most Common Compounds Of Sulfur
Sulfur is mostly used as a precursor to other chemicals. In around 85% of cases, sulfuric acid (H2SO4) is produced:
- 2 H2SO4 = 2 S + 3 O2 + 2 H2O
All living things require sulfur, however, it is almost invariably found in the form of organosulfur compounds or metal sulfides. Three amino acids (cysteine, cystine, and methionine) and two vitamins are found in organosulfur compounds (biotin and thiamine). Several cofactors contain sulfur, including glutathione, thioredoxin, and iron-sulfur proteins.
Disulfides, or S–S bonds, offer mechanical strength and insolubility to the protein keratin, which is found in the outer skin, hair, and feathers. Sulfur is a necessary macronutrient for all living things and one of the most critical chemical components in metabolic function.
Sulfur has three oxidation states: 2 (sulfide, S2), +4 (sulfite, SO32), and +6 (sulfite, SO32) (sulfate, SO42). It can be combined with nearly any other substance. Catenation—the bonding of an atom to another identical atom—is a rare feature of some sulfur compounds, second only to carbon.
As a result, sulfur atoms can form ring systems and chain structures. The most important sulfur compounds and chemical groups are listed here.
1. Hydrogen Sulfide
One of the most well-known sulfur compounds is hydrogen sulfide (H2S), often known as sulfuretted hydrogen or stinkdamp. The gas that gives rotten eggs their unique odor is colorless and extremely lethal. It is typically found in volcanic and mineral water vapors, and it is formed spontaneously when organic sulfur-containing molecules break down.
Large amounts of hydrogen sulfide are created when sulfur is extracted from petroleum. It was once frequently used in chemical laboratories as an analytical reagent. All metals, except gold and platinum, react with sulfur to form inorganic sulfides.
These sulfides, also known as hydrogen sulfide salts, are ionic compounds that include the negatively charged sulfide ion S2. Inorganic sulfides are major ores for iron, nickel, copper, cobalt, zinc, and lead.
2. Sulfur Oxides
Sulfur and oxygen combine to form a variety of oxides, the most notable of which is sulfur dioxide, a heavy, colorless, and toxic gas. It’s most commonly used as a precursor to sulfur trioxide (SO3) and consequently sulfuric acid (H2SO4). In the industry, it’s also employed as a bleach and a reducing agent.
3. Halides
Sulfur can form a variety of compounds when combined with halogen elements.
1. When Coupled With Chlorine
When coupled with chlorine, it creates sulfur chlorides, such as di sulfur dichloride, S2Cl2, a corrosive golden-yellow liquid used in the manufacture of chemical goods. When it reacts with ethylene, it creates mustard gas, and when it reacts with unsaturated acids produced by lipids, it forms greasy chemicals.

2. When Sulfur Combines With Fluorine
When sulfur combines with fluorine, it produces sulfur fluorides, the most useful of which is sulfur hexafluoride, or SF6, an insulator gas utilized in several electrical devices.
3. Oxyhalides
Sulfur can also be found in oxyhalides, which are molecules that include both oxygen and halogen atoms. The term thionyl is used to describe compounds that include the SO unit, while the term sulfuryl is used to designate compounds that contain SO2.
4. Thionyl Chloride
Thionyl chloride, also known as SOCl2, is a viscous, toxic, and volatile liquid that is used in organic chemistry to convert carboxylic acids and alcohols into chlorine-containing compounds.
5. Sulfuryl Chloride
Sulfuryl chloride, also known as SO2Cl2, is a liquid with similar physical properties that are used to create sulfur, chlorine, or both-containing chemicals.
Uses Of Sulfur
Sulfur may be broken down into 16 distinct acids that contain oxygen. Only four or five of these, however, have been generated in their most pure form. These acids, particularly sulfurous acid and sulfuric acid, are used extensively in the chemical industry.
Sulfuric acid (H2SO3) is generated when sulfur dioxide is combined with water. Its most important salt is sodium sulfite, often known as Na2SO3, a reducing agent used in the manufacture of paper pulp, photography, and the removal of oxygen from boiler feedwater. Sulfuric acid is one of the most valuable chemicals.

Water and sulfur Trioxide are used to manufacture fertilizers, pigments, dyes, pharmaceuticals, explosives, detergents, and inorganic salts and esters, among other things. Organic sulfur compounds are a diverse and significant group of organic substances.
Sulfur-containing amino acids (e.g., cysteine, methionine, and taurine) are examples of sulfur-containing amino acids that are key components of hormones, enzymes, and coenzymes. A range of pharmaceuticals (sulfa drugs, dermatological agents), insecticides, solvents, and agents used in the manufacturing of rubber and rayon are all key sources of synthetic organic sulfur compounds.
Summary
Sulfur is a chemical element having symbol S. It’s a nonmetallic, abundant, and multivalent substance. The production of sulfuric acid for sulfate and phosphate fertilizers is the most prevalent economic use of sulfur. Sulfur and oxygen combine to form a variety of oxides. Sulfur dioxide, a heavy, colorless, and toxic gas are the most important.
Physical Characteristics
Sulfur can be found in a wide range of polyatomic compounds. Octa sulfur, often known as cyclo-S8, is the most well-known allotrope. Octa sulfur is a soft, brilliant yellow solid with a match-like odor. It melts at 115.21 °C (239.38 °F), boils at 444.6 °C (832.3 °F), and sublimates easily.
Between melting and boiling temperatures, octa sulfur changes allotropes again, shifting from -octa sulfur to -sulfur, with lower density but increased viscosity due to the formation of polymers. Molten sulfur turns a dark red color at temperatures above 200 °C (392 °F).
Chemical Characteristics
When sulfur is burned with a blue flame, it creates sulfur dioxide, which has a suffocating and terrible odor. Sulfur can be dissolved in carbon disulfide and, to a lesser extent, other nonpolar organic solvents such as benzene and toluene, but not in water. The first and second ionization energies of sulfur are 999.6 and 2252 kJ/mol, respectively.
Despite these statistics, the +2 oxidation state is rather rare, with the +4 and +6 oxidation states being more common. The magnitude of the increase generated by electron transfer between orbitals is 4556 and 8495.8 kJ/mol for the fourth and sixth ionization energies, respectively; these states are only stable with strong oxidants such as fluorine, oxygen, and chlorine.
Occurrence In Nature
Sulfur compounds and elemental sulfur deposited by active volcanoes account for the majority of the yellow and orange colors. Sulfur is the fifth most plentiful element on Earth and the tenth most abundant element in the universe in terms of mass.
Sulfur is typically found in the form of sulfide and sulfate minerals on Earth, but it is also found in its pure, native form on rare occasions. Sulfur was known in ancient times because it was abundant in its natural state, and it was used in ancient India, Greece, China, and Egypt for many purposes.
Almost all elemental sulfur is now produced as a byproduct of sulfur-containing impurities being extracted from natural gas and petroleum. Manufacturing sulfuric acid for sulfate and phosphate fertilizers, as well as other chemical processes, is the most prevalent commercial use of the element.
Sulfur is found in matches, pesticides, and fungicides. Organosulfur compounds are used to make scented natural gas, skunk odor, grapefruit, and garlic, among other things. Hydrogen sulfide is responsible for the odor of decaying eggs and other biological processes.
S-32 is created by the fusion of one silicon nucleus and one helium nucleus at a depth where the temperature exceeds K-2.5109 inside big stars. Sulfur is the 10th most common element in the universe because it is produced by the alpha process, which produces abundant elements.
Sulfur is found in a variety of meteorites, usually in the form of sulfide. Carbonaceous chondrites have a sulfur content of up to 6.6 percent, which is higher than ordinary chondrites. Free sulfur, sulfates, and other sulfur compounds are found in carbonaceous chondrites.
In Terms Of Mass, It Is The Sixth Most Plentiful Element On The Earth.
In terms of mass, it is the sixth most plentiful element on the earth. Elemental sulfur can be found around hot springs and volcanic sites all over the world, particularly along the Pacific Ring of Fire; volcanic resources are mined in Indonesia, Chile, and Japan. The largest single crystal in these deposits measures 221611 cm and is polycrystalline.
During the Industrial Revolution, Sicily was a key supply of sulfur. At depths where the boiling point of water is higher than the melting point of sulfur, molten sulfur lakes up to 200 m in diameter have been observed on the seafloor, connected with undersea volcanoes.
Anaerobic bacteria working on sulfate minerals like gypsum produce native sulfur in salt domes. Significant salt dome deposits have been discovered along the Gulf of Mexico’s coast, as well as in eastern European and western Asian evaporites. To produce native sulfur, geological processes may be sufficient.
Until recently, commercial production relied on fossil-based sulfur resources from salt domes in the United States, Russia, Turkmenistan, and Ukraine. Commercial production is currently taking place in Poland’s Osiek mine. The majority of these sources are no longer used since they have little commercial value.
Some Other Derivatives Of Sulfur
Pyrite (iron sulfide), cinnabar (mercury sulfide), galena (lead sulfide), sphalerite (zinc sulfide), stibnite (antimony sulfide), and sulfate minerals including gypsum (calcium sulfate), alunite (potassium aluminum sulfate), and barite (potassium aluminum s (barium sulfate).
Major Applications
Sulfuric Acid (H2SO4)
-
Sulfur is mostly used as a precursor to other chemicals. Over 85% was converted to sulfuric acid (H2SO4) in 1989:
-
Sulfuric acid was first produced in the year 2000.
-
In 2010, more sulfuric acid was produced in the United States than any other inorganic industrial chemical. The principal use of the acid is to extract phosphate ores for fertilizer manufacture. Oil refinery, wastewater treatment, and mineral extraction all require sulfuric acid.
Other Applications Of Sulfur Chemistry
Sulfur reacts quickly with methane to form carbon disulfide, which is utilized in the production of cellophane and rayon. Sulfur is also used in the vulcanization of rubber, which involves the crosslinking of organic polymers by polysulfide chains.
Sulfites are commonly used to bleach paper and preserve dried fruit in big quantities. Many surfactants and detergents contain sulfate derivatives (for example, sodium lauryl sulfate). 100 million tons of calcium sulfate (CaSO4•2H2O), generally known as gypsum, are mined each year for use in Portland cement and fertilizers.
When silver-based photography was popular, sodium and ammonium thiosulfate were extensively used as “fixing agents.” Gunpowder contains sulfur (“black powder”).
Summary
Sulfur can be found in a wide range of polyatomic compounds. Octa sulfur, often known as cyclo-S8, is the most well-known allotrope. Sulfur has 23 known isotopes, four of which are stable, in addition to 35S. Sulfur ranks 10th among the most common elements in the universe. Elemental sulfur can be found in abundance around hot springs and volcanic sites around the world.
Sulfur As A Fertilizer
Sulfur is being used increasingly frequently in fertilizers. The most prevalent kind of sulfur used in fertilizers is calcium sulfate. Because elemental sulfur is hydrophobic, it cannot be used by plants (insoluble in water).
Over time, soil microorganisms can convert it to soluble derivatives, which can then be used by plants. Biologically generated sulfur particles are naturally hydrophilic and easier to distribute over land in a spray of diluted slurry due to a biopolymer coating, resulting in faster uptake.
Chemicals Of The Highest Quality
A molecular model exists for the insecticide malathion.
Uses Of Organosulfur Compounds
Organosulfur compounds are used in pharmaceuticals, dyes, and agrochemicals. Many pharmaceuticals include sulfur, notably antibacterial sulfonamides, generally known as sulfa drugs. Sulfur is found in many bacterial defense compounds. Most -lactam antibiotics, including penicillins, cephalosporins, and monobactams, include sulfur.
Magnesium Sulfate
Magnesium sulfate, often known as Epsom salts, can be used as a laxative, a bath additive, an exfoliant, a magnesium supplement for plants, or (when dried) as a desiccant when in hydrated crystal form.
Fungicides And Pesticides
Sulfur candles were originally sold for home fumigation. Elemental sulfur was one of the first fungicides and insecticides. Dusting sulfur, or elemental sulfur in powdered form, is a common fungicide for grapes, strawberries, a range of vegetables, and a variety of other crops. It can be used to treat a variety of powdery mildew infections, including black spots.
In organic farming, sulfur is the most important fungicide. It is the sole fungicide used in organically farmed apple production to treat the major disease apple scab when temperatures are colder. In these applications, sulfur (elemental sulfur created biologically with hydrophilic characteristics) can also be used.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or a dusting plane. Wettable sulfur is a brand name for dusting sulfur that has been made water-miscible by adding additional ingredients. It is used as a fungicide on plants and soil to combat mildew and other mold-related diseases and has similar purposes.
-
Elemental sulfur powder, an “organic” (i.e. “green”) insecticide, is used to control ticks and mites (really an acaricide). A common method of application is to dust the clothing or limbs with sulfur powder.
-
A diluted solution of lime sulfur is used to treat ringworm (fungi), mange, and various dermatoses and parasites (produced by mixing calcium hydroxide with elemental sulfur in water).
-
Sulfur candles, which were once used to fumigate buildings and wine barrels, are now deemed too unsafe for use in homes.
In The Winemaking And Food Preservation Industries, Bactericides Are Used.
Small amounts of sulfur dioxide gas (or equivalent potassium meta bisulfite) added to fermented wine to produce traces of sulfurous acid (formed when SO2 reacts with water) and its sulfite salts in the mixture have been named “the most potent instrument in winemaking.”
Similar techniques stretch back to antiquity, but the practice was only described in the fifteenth century. This method is used by both large industrial wine producers and small organic wine producers.
Sulfur dioxide and other sulfites have long been used in the food industry for their antibacterial and antioxidant properties. The practice has dropped since reports of some people experiencing an allergy-like reaction to sulfites in foods.
Pharmaceuticals
In pharmaceutical skin treatments, sulfur (specifically, octa sulfur, S8) is used to treat acne and other skin diseases. Precipitated sulfur and colloidal sulfur are used to treat acne vulgaris, acne rosacea, and seborrhoeic dermatitis as lotions, creams, powders, soaps, and bath additives. Dryness, stinging, itching, and peeling of the skin at the application site are all common side effects.
Summary
In fertilizers and other agrochemicals, sulfur is becoming more commonly used. Because elemental sulfur is hydrophobic (insoluble in water), plants cannot utilize it. However, soil bacteria may convert it to soluble derivatives, which can subsequently be used by plants.
Biological Function
Sulfur is an essential element in all living things. Sulfur is the eighth-most abundant element in the human body by weight, almost equal to potassium and slightly higher than sodium and chlorine.
Proteins And Organic Cofactors
In plants and animals, the amino acids cysteine and methionine contain the majority of sulfur, which is found in all polypeptides, proteins, and enzymes that contain these amino acids. Humans must ingest methionine, which is an important amino acid.
Methionine can be utilized to create cysteine and all sulfur-containing molecules in the human body, except the vitamins biotin and thiamine. The enzyme sulfite oxidase is essential for the metabolism of methionine and cysteine in humans and animals.
Disulfide Bonds (S-S bonds)
Disulfide bonds (S-S bonds) between cysteine residues in peptide chains are essential for protein structure and assembly. Extra toughness and rigidity are provided by these covalent connections between peptide chains. Feathers and hair, for example, have a significant amount of S-S bonds with cysteine and sulfur, which contributes to their strength.
Eggs are high in sulfur to help chicks develop feathers, and the odor of rotting eggs is caused by hydrogen sulfide. Hair and feathers’ high disulfide bond content contributes to their indigestibility and characteristic disagreeable odor when burned.
Precautions Needed To Use Sulfur Compounds
Most soluble sulfate salts, such as Epsom salts, are non-toxic, as is elemental sulfur. Aluminum sulfate is used in the purification of drinking water, wastewater treatment plants, and papermaking. Soluble sulfate salts are poorly absorbed and laxative. When injected parenterally, they are freely filtered by the kidneys and eliminated with very little toxicity in multi-gram amounts.
Sulfur dioxide is produced when sulfur is burned in the air. This gas creates sulfurous acid and sulfites when it comes into contact with water; sulfites are antioxidants that prevent the growth of aerobic bacteria and can be used as a food additive in tiny amounts. These acids can injure the lungs, eyes, and other tissues when present in high amounts.
Sulfite in high concentrations stops breathing in species without lungs, such as insects and plants. In the presence of water, sulfur trioxide (produced by catalysis from sulfur dioxide) and sulfuric acid are both very acidic and corrosive. Sulfuric acid is a powerful dehydrator that can strip sugar and organic tissue of available water molecules and water components.
FAQS
Mostly Asked Questions are given below.
1. Why is it that sulfur has only 6 valence electrons?
The orbitals associated with an atom’s highest occupied energy level include valence electrons. The remaining electrons, referred to as inner shell electrons, are not involved in bonding and so are not worth studying. Sulfur contains six valence electrons as a result.
2. Is the octet rule usually followed by sulfur?
As in the molecule SF2, sulfur can follow the octet rule. Each atom has eight electrons surrounding it. It is possible to sufficiently excite the sulfur atom to force valence atoms into the d orbital, allowing compounds like SF4 and SF6 to form.
3. Is it possible for sulfur to receive or lose electrons?
Because a regular Sulfur atom has six valence electrons, we may deduce that the atom would like two extra electrons to fill its outer shell using Octet’s Rule. With the sulfur atom, these oxygen atoms form a covalent double bond.
4. Is it possible for nitrogen to enlarge its octet?
Atoms having an octet expansion
Because they are in the third period, phosphorus has 5 orbitals (10 electrons) and sulfur has 6 orbitals (12 electrons), but nitrogen and oxygen can never have expanded octets because they are in the second period and there is no such thing as a 2d orbital.
5. What is the proper electron configuration for the sulfur ion?
Sulfur’s ion will have the correct electron configuration of 1s2, 2s2, 2p6, 3s2, 3p6.
6. What is the most likely ion for sulfur to form?
To reach a full octet of eight electrons, a neutral sulfur atom will need to gain two electrons. As a result, we expect the most frequent charge on a sulfur ion to be 2.
7. Is the atom sulfur a neutral one?
Elemental sulfur, or sulfur in a neutral element, possesses six electrons and is hence a neutral element. Because S2 ions have 8 electrons, they have a 2-minus charge.
8. How can you find oxygen’s valence electrons?
The number of valence electrons in neutral atoms is equal to the atom’s main group number. The main group number of an element can be found in its periodic table column. Carbon, for instance, belongs to group 4 and has four valence electrons. Oxygen belongs to group 6 and has a valence electron count of 6.
9. What is the distinction between outer and valence electrons?
The amount of electrons in an atom’s outermost shell impacts its reactivity or its proclivity for forming chemical connections with other atoms. The valence shell is the outermost shell, and the electrons in it are known as valence electrons.
10. Why do valence electrons become excited so easily?
Due to the comparatively low energy required to remove the extra valence electrons, atoms with one or two valence electrons more than a closed shell are extremely reactive.
Conclusion
Valence electrons are electrons that are present in an atom’s outermost shell. An atom’s stability can be achieved by adding or losing electrons for the entire outer shell. Sulfur’s valency could be as high as 6, depending on its configuration of electrons. Sulfur is a chemical element that is not metallic. The most reactive chemical element is sulfur.
Related articles
1. Carbon dioxide molecule
2. How many electrons are in sulfur?
3. Sulfurous acid
4. Oxidizing agent
5. Sulfur Protons Neutrons Electrons
