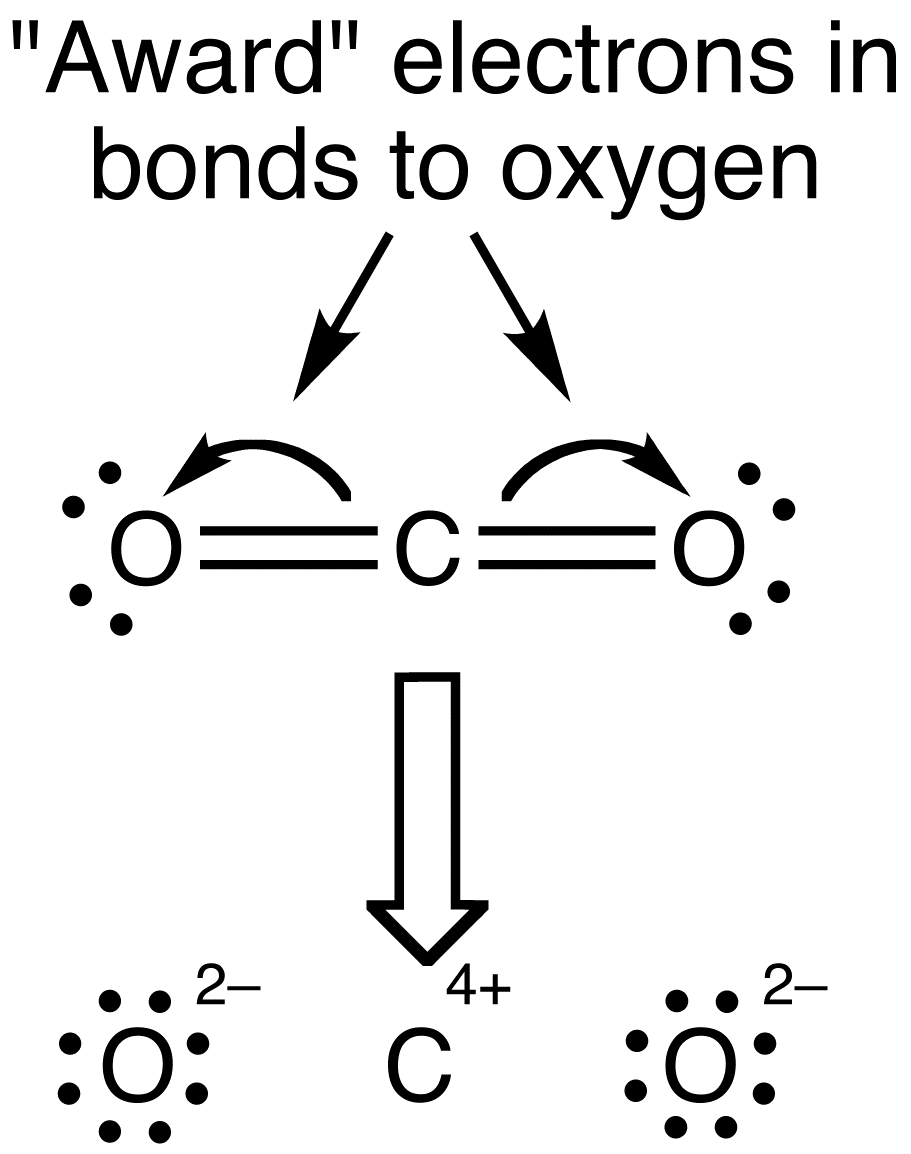
Lewis Dot Structure For CO2 consist of Carbon which has four valence electrons, which form four bonds in total. So, carbon is occupied by four dots. Only two bonds are required for oxygen, which are depicted by the lone dots to the left and right of the O atoms. The dots above and below the O’s do not meet.

CO2 Lewis Dot Structure
The main thing noticed at first glance is the carbon at the middle of the CO2 Lewis structure. A bond is formed by the O atoms to the C atom. There will be no direct connection between the Oxygen atoms. Carbon is almost always in the center, and the other atoms are connected to it.
Second, meet the lone dots on each Oxygen atom to the central Carbon. Each O must be bonded twice. On the other hand, Carbon needs four bonds. So, Carbon forms two bonds with each Oxygen atm.
Double CO2 Bond
The bonds between the C and O atoms appear as parallel lines. These are referred to as double bonds. Four dots and two sticks or lines surround each O, symbolizing another four electrons in its double bond.
As a result, each O has an octet and is stable because it is surrounded by 8 total valence electrons. Carbon has four bonds, two of which are double bonds in this example. As a result, carbon contains eight valence electrons.
What Is Carbon Dioxide And How Does It Affect You?
| 1. Chemical formula | CO2 |
|---|---|
| 2. Molar mass | 44.01 g/mol |
| 3. Density | 1562 kg/m3 |
| 4. Appearance | Gas with a colorless appearance |
| 5. Low concentrations | no odor; high concentrations: harsh and acidic |
| 6. Molar mass | 44.01 |
Occurrence Of Carbon Dioxide
Because carbon dioxide is soluble in water, it can be found in groundwater, rivers and lakes, ice caps, glaciers, and the ocean. Petroleum and natural gas reserves contain it. Carbon dioxide has a pungent, acidic odor and tastes like soda water in the mouth. At ordinary concentrations, it is, however, odorless.
The current concentration is roughly 0.04 percent (412 parts per million) by volume, up from 280 parts per million prior to the industrial revolution. Natural sources include volcanoes, forest fires, hot springs, and geysers, and it is liberated from carbonate rocks by dissolving in water and acids.
Importance Of CO2
Carbon dioxide is the primary source of accessible carbon in the carbon cycle, and its concentration in the pre-industrial atmosphere has been regulated by photosynthetic organisms and geological events since late in the Precambrian.
Photosynthesis is a process in which plants, algae, and cyanobacteria use sunlight to synthesize carbohydrates from carbon dioxide and water, with oxygen as a byproduct.
When aerobic organisms digest organic molecules for energy, oxygen is consumed and CO2 is generated as waste. As CO2 is needed for photosynthesis in plants and humans and animals rely on plants for nutrition, CO2 is essential for life on Earth.
What Causes CO2 To Form?
It is returned to the water by fish gills, and it is returned to the air by air-breathing terrestrial organisms, including humans. Carbon dioxide is manufactured during the manufacturing process.
-
In the manufacture of bread, beer, and wine, carbon dioxide is created by the breakdown of organic components and the fermentation of carbohydrates.
-
It is produced from the combustion of wood, peat, and other organic materials, as well as fossil fuels such as coal, petroleum, and natural gas.
-
It’s an unwelcome consequence of a number of large-scale oxidation processes, including the production of acrylic acid (which produces over 5 million tons per year).
Applications Of Carbon dioxide
It’s being industrial material is used in welding and fire extinguishers, as a pressurizing gas in air guns and oil recovery, as a chemical feedstock. It is used to make drinking water and carbonated beverages like beer and sparkling wine effervescent. Dry ice is a refrigerant and abrasive made up of a frozen solid form of CO2.
Summary
Carbon dioxide (CO2) is a colorless, acidic gas with a density that is around 53% higher than that of dry air. Because it is soluble in water, it can be found in groundwater, rivers and lakes, ice caps, glaciers, and the ocean.
Carbon dioxide is produced during the manufacturing process. It’s an unwelcome consequence of a number of large-scale oxidation processes, including the production of acrylic acid.
What Role Does CO2 Play In Our Environment?
It’s a versatile industrial material that’s used in welding and fire extinguishers, as a pressurizing gas in air guns and oil recovery, as a chemical feedstock.
1. Carbon dioxide is the most abundant long-lived greenhouse gas in the Earth’s atmosphere.
2. Since the Industrial Revolution, anthropogenic emissions have increased their concentration in the atmosphere, resulting in global warming.
3. It is used as a raw material in the manufacturing of fuels and chemicals.
4. In the Earth’s atmosphere, carbon dioxide is the most prevalent long-lived greenhouse gas.
5. Anthropogenic emissions, mostly from the use of fossil fuels and deforestation, have drastically increased their concentration in the atmosphere since the Industrial Revolution, resulting in global warming.
6. Because carbon dioxide dissolves in water and forms carbonic acid, it contributes to ocean acidification.
CO2 Molecular Structure
As a linear triatomic molecule, CO2 has four vibrational modes, as illustrated in the diagram. In the symmetric and antisymmetric stretching modes, the atoms move along the molecule’s axis.
Because of the symmetry of the molecule, there are two degenerate bending modes, meaning they have the same frequency and energy.
The frequency of the two bending modes can differ due to the varied interactions between a molecule and a surface or another molecule. Two vibrational modes visible in the infrared (IR) spectrum are the antisymmetric stretching mode at wavenumber 2349 cm1 (wavelength 4.25 m) and the degenerate pair of bending modes at 667 cm1 (wavelength 15 m).
The symmetric stretching mode cannot be used since it does not form an electric dipole. It can be detected by Raman spectroscopy at 1388 cm1 but not by IR spectroscopy (wavelength 7.2 m). The vibrational movements of carbon dioxide molecules in the gas phase are significant, and they do not keep a constant shape.
Reaction Capability Of CO2
No molecules in the gas phase are ever fully linear, according to this experiment and theoretical calculations based on an ab initio potential energy surface of the molecule.
When combined with water in an aqueous solution,
1. In A Watery Solution
Carbon dioxide is soluble in water, where it reversibly converts to H2CO3 (carbonic acid), a weak acid due to incomplete ionization in water.
CO2 + H2O→ H2CO3
Summary
In the Earth’s atmosphere, carbon dioxide is the most prevalent long-lived greenhouse gas. Because it dissolves in water and creates carbonic acid, it contributes to ocean acidification.
As a linear triatomic molecule, CO2 has four vibrational modes, as illustrated in the diagram. Carbon dioxide is soluble in water, where it undergoes a reversible process to produce hydrogen.
2. Carbonic Acid Is A Type Of Acid.
Due to its diprotic nature, carbonic acid has two acid dissociation constants: the first is for dissociation into the bicarbonate (also known as hydrogen carbonate) ion (HCO3):
H2CO3→ HCO3_ + H+
At 25 °C, K a1 = 2.5104 mol/L, and pK a1 = 3.6.
Because most of the dissolved CO2 remains as CO2 molecules, Ka1(apparent) has a much larger denominator and a much lower value than true Ka1.
Bicarbonate Ion
The Molecule Of Carbon Dioxide
The bicarbonate ion is an amphoteric species that can act as an acid or a base depending on the pH of the solution. At high pH, it breaks into the carbonate ion (CO32):
MR + CO2→ RCO2M
pKa2 = 10.329; Ka2 = 4.691011 mol/L
Carbonic anhydrase is an enzyme in living organisms that catalyzes the creation of carbonic acid.
CO2 Chemical Reactions
CO2 is a strong electrophile with electrophilic reactivity that is similar to that of benzaldehyde and strong, -unsaturated carbonyl compounds. Nucleophiles with CO2, on the other hand, have a lower thermodynamic favorability than electrophiles with comparable reactivity and are commonly found to be exceedingly reversible.
To form carboxylates, only extremely potent nucleophiles, such as Grignard reagent carbanions and organolithium compounds, interact with CO2:
-
RCO2M → MR + CO2
-
Brand R = alkyl or aryl, where M = Li or Mg
Metals And CO2
In metal carbon dioxide complexes, CO2 acts as a ligand, making it easier to convert CO2 to other molecules. Normally, turning CO2 to CO is a time-consuming and complicated process:
CaCO3+2HCl→ CaCl2+H2CO3
CO2 Reaction In Plants
Plants and cyanobacteria are photoautotrophs, which means they use the sun’s energy to photosynthesize simple sugars from CO2 in the air and water:
This reaction, which is mediated by the nickel-containing enzyme carbon monoxide dehydrogenase, has a redox potential of around 0.53 V when compared to a standard hydrogen electrode at pH 7.
Characteristics Of CO2
The physical characteristics of CO2 are given below:
1. Solid carbon dioxide in the form of dry ice pellets is a popular choice.
2. Carbon dioxide is colorless.
3. When employed in tiny volumes, the gas is odorless, but at sufficiently large concentrations, it emits a harsh, acidic odor.
4. At standard temperature and pressure, carbon dioxide has a density of 1.98 kg/m3, which is about 1.53 times that of air.
5. At temperatures and pressures above the critical point, carbon dioxide behaves as a supercritical fluid, which is known as supercritical carbon dioxide.
Isolation And Production
Although it is feasible to distil carbon dioxide from the air, it is inefficient. Carbon dioxide is largely an unrecoverable waste product produced in a variety of ways at various scales in the industrial sector.
When carbon-based fuels are burned, they emit carbon dioxide and, with the exception of pure carbon, water. These fuels include methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), coal, wood, and general organic matter.
Take the following chemical reaction between methane and oxygen, for example:
CO2 + 2 H2O = CH4 + 2 O2
In the summer of 2018, numerous ammonia facilities were temporarily shut down for repair, resulting in a carbon dioxide scarcity for these purposes across Europe. When metal carbonates are subjected to acids, they release CO2. As a result, it is possible to collect it directly from natural carbon dioxide springs, where acidified water combines with limestone or dolomite to produce it.
The reaction between hydrochloric acid and calcium carbonate (limestone or chalk) is depicted in the diagram below:
2 HCl + CaCO3 → CaCl2 + H2CO3
It converts into CO2 and water after that:
CO2 + H2O → H2CO3
The reaction is accompanied by foaming or bubbling, or both, as the gas is released. They are commonly employed in industry because they can neutralize waste acid streams.
Brewing produces carbon dioxide as a by-product of the sugar fermentation process.
2 CO2 + 2 C2H5OH → C6H12O6
Anaerobic organisms decompose organic matter to create methane, carbon dioxide, and other compounds. Regardless of the type of organic matter, the production of gases follows a well-defined kinetic pattern. About 40–45 percent of the gas emitted during landfill decomposition (dubbed “landfill gas”) is carbon dioxide. The remaining 50–55 percent is mostly made up of methane.
Summary
At standard temperature and pressure, carbon dioxide has a density of 1.98 kg/m3, which is about 1.53 times that of air. At low concentrations, the gas is odorless, but at sufficiently high concentrations, it emits a pungent, acidic stench. Brewing produces carbon dioxide (CO2) as a by-product of the sugar fermentation process.
Applications
Carbon dioxide is used in the food business, the oil industry, and the chemical industry. The chemical has a variety of commercial uses, but one of the most important is in the production of carbonated beverages; it is responsible for the sparkle in soda water, beer, and sparkling wine.
1. Precursor Of Chemicals
Carbon dioxide is mostly used in the chemical industry to produce urea, with a small amount being used to produce methanol and a number of other compounds. Several carboxylic acid derivatives, such as sodium salicylate, are made using the Kolbe-Schmitt process.
In addition to classic CO2 processes for chemical synthesis, electrochemical approaches are being examined at the research level. In particular, the use of renewable energy for the production of biomass-based fuels.
The use of renewable energy to produce CO2 fuels (such as methanol) is tempting because it could result in fuels that are easy to transport and use within present combustion technologies while releasing no net CO2.
2. Agriculture
Photosynthesis in plants requires the presence of carbon dioxide. Greenhouse atmospheres may (and, if large enough, must) be enriched with additional CO2 to maintain and accelerate plant development.
Because very high concentrations of carbon dioxide (100 times atmospheric concentration or greater) can be harmful to animal life, raising the concentration to 10,000 ppm (1 percent) or higher for many hours will kill pests like whiteflies and spider mites in a greenhouse.
3. Foods
CO2 is utilized in the production of the following foods:
-
Carbon dioxide is a food component that is used as a propellant and acidity control in the food industry. In Europe, it is available for use.
-
The use of renewable energy to produce CO2 fuels (such as methanol) is tempting because it could result in fuels that are easy to transport and use within present combustion technologies while releasing no net CO2.
-
Candy that has been compressed with carbon dioxide gas is known as Pop Rocks.
-
Because leavening agents produce carbon dioxide, the dough rises.
-
When heated or subjected to acids, chemical liveners like baking powder and baking soda release carbon dioxide, whereas baker’s yeast produces carbon dioxide by digesting carbohydrates within the dough.
4. Beverages
Carbon dioxide is used to manufacture carbonated soft drinks and soda water. Natural fermentation has traditionally been used to carbonate beer and sparkling wine, although many producers now use carbon dioxide recovered during the fermentation process.
The most common technique of carbonation in bottled and kegged beer is recycled carbon dioxide. Except for British real ale, draught beer is generally transported from kegs in a cold room or basement to dispensing faucets on the bar using pressurized carbon dioxide, which is occasionally combined with nitrogen.
The flavor of soda water (and similar taste sensations in other carbonated beverages) is caused by dissolved carbon dioxide rather than the gas’s bursting bubbles. Carbonic anhydrase 4 converts carbonic acid to carbonic acid, which produces a sour taste and a tactile sensation from the dissolved carbon dioxide.
5. Winemaking
After harvest, dry ice is used to keep grapes fresh. Carbon dioxide in the form of dry ice is widely used during the cold soak phase of winemaking to quickly freeze clusters of grapes after picking to help avoid spontaneous fermentation by wild yeast.
The most significant advantage of using dry ice instead of water ice is that it cools the grapes without adding any additional water, which may reduce the sugar level in the grape must and, as a result, the alcohol concentration in the finished wine.
CO2 As Pressurized Gas
Carbon dioxide is one of the most commonly used compressed gases in pneumatic (pressurized gas) systems in portable pressure tools. Although it oxidizes most metals in the welding arc, carbon dioxide is also used as a welding environment.
Despite evidence that carbon dioxide welds are more brittle than welds done in more inert atmospheres, carbon dioxide is widely used in the automotive industry. Because CO2 may react at these high temperatures, it is also referred to as MAG welding, or Metal Active Gas, when used for MIG welding.
It produces a hotter puddle than inert atmospheres, allowing for better flow qualities. However, this could be due to air processes at the puddle site. This has the reverse effect of what is desired during welding, since it tends to embrittle the place, however this isn’t always a concern.
When welding, this is usually the opposite of the intended effect because it tends to embrittle the site, while it may not be an issue for typical mild steel welding when final ductility isn’t a major concern.
CO2 As A Preservative
Carbon dioxide is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and it transitions from gas to liquid at room temperature at an attainable pressure of approximately 60 bar (870 psi; 59 atm), allowing more carbon dioxide to fit in a given container than would be possible otherwise.
Life jackets often have pressurized carbon dioxide canisters for quick inflation. CO2 capsules can also be used to fill air guns, paintball markers/guns, inflate bicycle tires, and make carbonated water.
Carbon Dioxide In Liquid Form Is Used In A Variety Of Applications.
Carbon dioxide can also be used to kill pests in large quantities. Liquid carbon dioxide is used in supercritical drying of some foods and technical materials, specimen preparation for scanning electron microscopy, and decaffeination of coffee beans.
Carbon dioxide can also be used to kill pests in large numbers. Liquid carbon dioxide is used in supercritical drying of some foods and technical materials, specimen preparation for scanning electron microscopy, and decaffeination of coffee beans.
Summary
Carbon dioxide is also utilized to create a hypoxic environment for carbonic maceration, which is the method used to make Beaujolais wine. CO2 is one of the most commonly used compressed gases in pneumatic (pressurized gas) systems in portable pressure tools.
Because it is affordable and nonflammable, carbon dioxide is employed in many consumer items that require pressurized gas.
Use Of CO2 Fire Extinguishers
By filling the region around the flame with carbon dioxide, flames can be extinguished. By moving the flame, it deprives it of oxygen rather than extinguishing it. A pressurized liquid carbon dioxide is contained in some fire extinguishers, notably those designed for electrical fires.

Carbon dioxide extinguishers are effective against small flammable liquid and electrical fires, but they are ineffective against larger combustible fires because they do not significantly cool the burning substances, and once the carbon dioxide has dissipated, they can catch fire when exposed to atmospheric oxygen. It’s most typical to find them in server rooms.
-
Carbon dioxide has also been used as an extinguishing chemical in stationary fire-fighting systems for localized threats and total flooding of a protected zone.
-
International Maritime Organization regulations acknowledge carbon-dioxide systems for fire protection of ship holds and cargo spaces.
Multiple deaths have been linked to carbon-dioxide-based firefighting systems, as high levels of the gas can cause suffocation. An analysis of CO2 systems found 51 mishaps between 1975 and the study’s date (2000), resulting in 72 deaths and 145 injuries.
Supercritical CO2 Is Utilized As A Solvent.
Another word for supercritical carbon dioxide is supercritical carbon dioxide.
-
Liquid carbon dioxide is used to decaffeinate coffee because it is a good solvent for many lipophilic chemical compounds.
-
Carbon dioxide has piqued attention in the pharmaceutical and chemical processing industries as a safer alternative to traditional solvents such as organochlorides.
-
It’s also used by some dry cleaners for the same purpose (see green chemistry). Supercritical carbon dioxide is used in the production of a variety of aerogels due to its properties.
Medical And Pharmacological Applications
In medicine, up to 5% carbon dioxide (130 times atmospheric concentration) is added to oxygen to promote breathing after apnea and maintain a stable O2 level.
Energy
Extraction Of Fossil Fuels
For greater oil recovery, carbon dioxide is poured into or near producing oil wells under supercritical conditions, when it becomes miscible with the oil. This approach can increase original oil recovery by reducing residual oil saturation by 7% to 23% in addition to primary extraction.
When dissolved in subsurface crude oil, it acts as a pressurizing agent and reduces viscosity and modifies surface chemistry, allowing the oil to flow more quickly through the reservoir and to the removal well. Large pipe networks convey carbon dioxide to injection locations in mature oil fields.
Enhanced coal bed methane recovery would inject carbon dioxide into the coal seam to replace methane rather than depending on the removal of water (to reduce pressure) to make the coal seam release methane.
Biomass to fuel is a process that involves converting biomass into fuel:
CO2 from power plants might be poured into ponds to promote algae growth, which could then be converted to biodiesel fuel. A strain of the cyanobacterium Synchronous elongatus has been genetically engineered to allow it to produce the fuels is butyraldehyde and iso-butanol from CO2.
CO2 As A Refrigerant
Pressure-temperature phase diagrams of carbon dioxide (red) and water (blue) as a log-line chart exhibiting phase transitions at 1 atmosphere.
-
Carbon dioxide is a key refrigerant in the food industry, where it is used in the shipping and storage of ice cream and other frozen foods in both liquid and solid form.
-
Dry ice is a form of carbon dioxide that is utilized for small shipments when refrigeration isn’t possible.
-
Regardless of air temperature, solid carbon dioxide is always below 78.5 °C (109.3 °F) at standard atmospheric pressure.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the adoption of dichlorodifluoromethane (R12, a chlorofluorocarbon (CFC) compound).
Because 1,1,1,2-tetrafluoroethane (R134a, a hydrofluorocarbon (HFC) molecule) contributes more to climate change than CO2, CO2 may see a comeback. CO2 is ideal for cooling, refrigeration, and heating because of its physical properties, which include a high volumetric cooling capacity.
CO2 Helps To Keep Blood Pressure In Check.
Carbogen, an inhalable gas with a range of medical and research applications, is made by mixing carbon dioxide with up to 50% oxygen. The mofette, which are dry spas that use post-volcanic discharge carbon dioxide for medicinal purposes, is another medical application.
Applications Of Minor Importance
A laser that emits carbon dioxide is known as a carbon-dioxide laser. Carbon dioxide is used as the lasing medium in carbon-dioxide lasers, which are one of the oldest types of lasers. By continuously adding gas to the water and preventing the pH from rising, carbon dioxide can be used to keep the pH of swimming pools under control.
One of the advantages of this is that it eliminates the need to handle (more hazardous) acids.
Similarly, it is frequently used in calcium reactors to temporarily lower the pH of the water as it passes through calcium carbonate, allowing the calcium carbonate to dissolve more easily into the water, where it is used by some corals to build their skeleton.
The primary coolant in the United Kingdom’s advanced gas-cooled reactor for nuclear power generation. Carbon dioxide induction is frequently used to euthanize laboratory research animals. Two methods for administering CO2 are to place animals immediately into a closed, prefilled chamber containing CO2 or to expose them to a gradually increasing concentration of CO2.
According to the American Veterinary Medical Association’s 2020 standards for carbon dioxide induction, a displacement rate of 30 percent to 70% of the chamber or cage volume per minute is suitable for the humane euthanasia of tiny rodents. CO2 concentrations differ per species, with optimal percentages for minimal distress found.
As A Result Of Human Activities, Carbon Dioxide Levels Have Grown
1. Fossil Fuel Extraction And Combustion
CO2 concentrations in the atmosphere have increased by around 50% from the dawn of the industrial age to the year 2020 as a result of the extraction and burning of fossil fuels, which uses carbon that has been trapped in the lithosphere for millions of years.
The combustion of coal, petroleum, and natural gas produces the majority of CO2 created by human activities. Other major anthropogenic causes include cement production, deforestation, and biomass burning. Currently, around half of the carbon dioxide released by burning fossil fuels remains in the atmosphere, un-absorbable by plants or seas.
Volcanoes produce only 0.2 to 0.3 billion tons of CO2 per year, whereas human activities produce approximately 30 billion tons (9 billion tons of fossil carbon). As a result of human activity, CO2 levels have risen to levels not seen in hundreds of thousands of years.
Physiology Of Humans
Transport Of Blood
CO2 is delivered in three ways through the bloodstream. Depending on whether the blood is arterial or venous, the percentages alter.
1. 5–10% of the drug is dissolved in the plasma.
2. 5–10% of hemoglobin is made up of carbamine compounds.
Hemoglobin, the principal oxygen-carrying molecule in red blood cells, transports both oxygen and carbon dioxide. On the other hand, CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it attaches to the N-terminal groups of the four globin chains.
Due to allosteric effects on the hemoglobin molecule, CO2 binding reduces the amount of oxygen bound for a given partial pressure of oxygen. The Haldane Effect is a mechanism that helps carbon dioxide move from the tissues to the lungs.

Frequently Asked Questions
These are some of the queries that people may have.
1. What goes into the creation of a molecule?
When atoms join and form bonds, molecules are produced. A hydrogen molecule, abbreviated as H2, is formed when two hydrogen atoms join together.
2. Can you provide me a molecular example?
Carbon and hydrogen molecules make up the most basic organic compounds. In the molecule methane, one carbon is bonded to four hydrogens. Ethane is another example of a simple hydrocarbon.
3. Is carbon an atom or a molecule?
Carbon (from Latin: carbo “coal”) is a chemical element with atomic number 6 and the symbol C. It’s nonmetallic and tetravalent, meaning it’s made up of four electrons.
4. When atoms come together to form molecules, what happens?
When two or more atoms chemically bind together, a molecule is formed. In a covalent bond, electrons are shared between atoms. In a water molecule, covalent bonds occur between the two hydrogen atoms and the oxygen atom. As the name implies, a metallic bond occurs when two metallic substances come together.
5. How does a molecule appear?
Compound molecules are made up of atoms that come from two or more different elements. Water (H2O) has three atoms: two hydrogen (H) atoms and one oxygen (O) atom (O). Methane (CH4) is a common greenhouse gas that contains five atoms: one carbon and four hydrogens.
6. What three sorts of molecules are there?
Some examples of common molecules are as follows:
-
Hydrogen peroxide (H2O) (water)
-
N2 is a kind of gas (nitrogen)
-
The oxygen molecule O3 is a kind of oxygen (ozone)
-
Calcium Oxide (CaO) is a mineral that is found in nature (calcium oxide)
-
The chemical compound C6H12O6 has the formula C6H12O6 (glucose, a type of sugar)
-
Sodium chloride (NaCl) is a kind of salt (table salt)
7. Is it possible to make an atom disappear?
There are no atoms that have been destroyed or created. The bottom line is that matter can take many different forms throughout the universe. During any physical or chemical process, matter does not appear or disappear. Atoms created in the stars make up every living and nonliving object on Earth, including you (a very, very long time ago).
8. Which of the following molecules is the most common?
While atomic hydrogen (H2) is the most numerous molecules in the universe, "protonated molecular hydrogen," or H3+, is the second most abundant.
9. What examples do you have of complicated molecules?
Complex molecules are chemical compounds that take the form of multiple molecules. Examples of well-known substances include water (H2O) and carbon dioxide (CO2). Ionic compounds like sodium chloride are not the same as these chemicals (NaCl).
10. What’s the difference between ionic compounds and covalent compounds?
Ionic compounds are made up of atoms that have been bonded together by exchanging electrons, whereas covalent compounds are pure substances made up of atoms that have been joined together by exchanging electrons. Ionic compounds form when metals and non-metals interact, whereas molecular compounds develop when two non-metals interact.
11. Why does a single glucose molecule require six carbon dioxide molecules to produce?
Because carbon dioxide has one carbon per molecule and glucose molecules have six, it takes six carbon dioxide (CO2) molecules to generate one glucose molecule (C6H12O6).
12. What happens to CO2 during the Calvin cycle?
Carbon dioxide molecules are linked together with electrons and Hs from NADPH to make glucose. The exchange of O2 and CO2 takes place. It promotes their communication by allowing for easy dispersal.
13. Is CO2 oxidized or reduced in the Calvin cycle?
Carbon dioxide in the atmosphere is converted to glucose in the Calvin-Benson cycle. This involves employing the electrons generated by NADPH oxidation to reduce total CO2. CO2 is converted to glucose, while NADPH is converted to NADP+.
Conclusion
CO2 is a colorless, acidic gas with a density about 53% higher than that of dry air. A carbon atom is covalently connected to two oxygen atoms in a carbon dioxide molecule. It exists as a trace gas in the Earth’s atmosphere. In the mouth, carbon dioxide has a harsh, acidic odor and tastes like soda water.
It is, however, odorless at ordinary doses. CO2 is necessary for life on Earth because plants require it for photosynthesis and humans and animals rely on plants for nutrition. Carbon dioxide is a source of accessible carbon in the carbon cycle.
