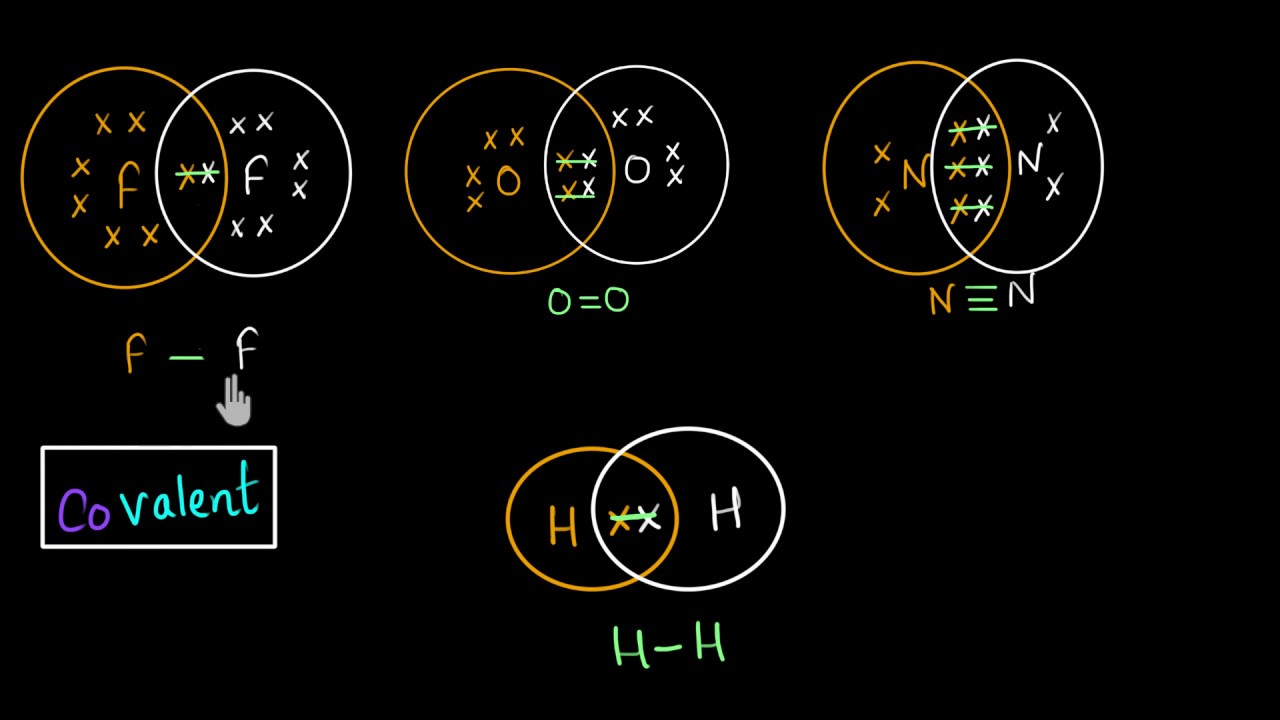
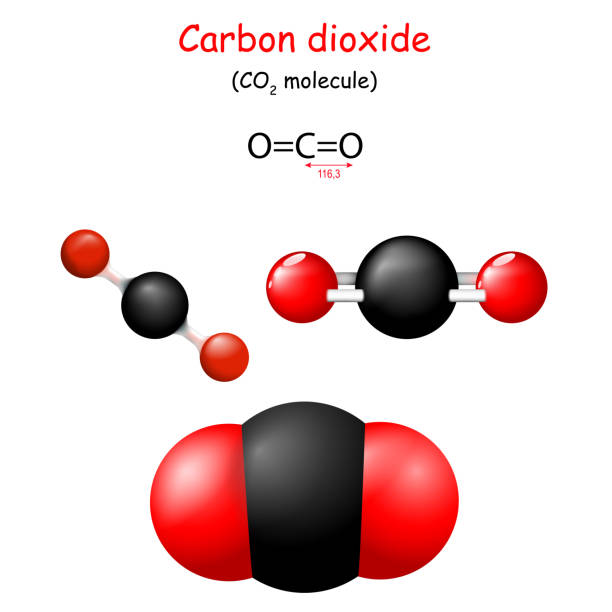
Electron dot structures of co2 determine the nature of bonds and atoms in molecule connected by it. Carbon dioxide is a colorless and odorless gas found naturally. CO2 is the chemical compound’s formula.
 Electron dot structures of co2
Electron dot structures of co2
When first learning about Lewis structure and Molecular Geometry, an excellent place to start is with Carbon Dioxide, first-time students who want to understand the principles of Lewis dot structures and how to draw them should begin with this molecule.
There are two atoms in CO2 or Carbon Dioxide: Carbon and Oxygen. Although it’s a contributor to the greenhouse effect and global warming, this gas has several applications in various fields.
 Electron-dot structures of CO2
Electron-dot structures of CO2
-
This molecule’s core atom is carbon.
-
The six valence electrons in the oxygen atom form two lone pairs.
-
A double bond must be formed because it only has one carbon atom to work with.
*, Unlike other elements, Carbon has no lone pairs of electrons.
-
Because of this, each oxygen atom is bound to it twice.
-
It has a significant impact on the emergence of life.
-
Gas is also an essential factor in the photosynthesis process.
 The molecular structure of CO2
The molecular structure of CO2
| Name of the Molecule | Carbon Dioxide |
|---|---|
| Molecular Formula | CO2 |
| Hybridization Type | sp |
| Bond Angle | 180° |
| Geometry | Linear |
The carbon and oxygen atoms in carbon dioxide form two double bonds. Two sigma and two pi bonds make up each double bond. Therefore carbon dioxide has four sigma and four pi bonds. Molecule-level information can be used to draw a Lewis or electron dot structure.
There are two Carbon and two oxygen atoms in carbon dioxide, which means the bond angle between them is 180 degrees. The carbon atom undergoes sp Hybridization, while sp2 hybridizes the two oxygen atoms.
Because Oxygen is more electronegative than Carbon, the two bonds created are polar, but the molecule as a whole is not polar because of the dipole moment cancellation.
 Carbon dioxide’s physical and chemical properties
Carbon dioxide’s physical and chemical properties
-
Carbon dioxide’s chemical formula is CO2.
-
Carbon dioxide is a colorless and odorless gas that is found naturally.
-
To maintain life on Earth, it is essential.
-
Even though it is only present in small amounts, this gas is essential.
Summary
Carbon dioxide contains four electrons, two of which come from Oxygen and two from Carbon. The octet rule is applicable since all four electrons are counted in carbon and oxygen octets.
 The CO2 Lewis dot structure
The CO2 Lewis dot structure
The first step is to determine the number of valence electrons in it. In CO2, the outer shell electrons that can participate in creating chemical bonds are shown by the valence electrons of CO2. Carbon and Oxygen’s valence electrons are shown as dots on the lewis diagram.
 CO2 total valence electron count
CO2 total valence electron count
Carbon and Oxygen’s valence electrons can be found using a periodic table. According to the occasional chart, Carbon is in 14 groups, and Oxygen is in 16. Carbon contains four valence electrons, whereas Oxygen has six.
-
Oxygen has a valence electron of six [Periodic group of Oxygen = 16].
-
There are 14 periodic groups in Carbon, and each one has a valence electron of four.
-
Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons
 Placing a minor electronegative atom
Placing a minor electronegative atom
If Carbon or Oxygen has the lowest electronegativity, we should position it in the center of lewis’s diagram. From left to right, electronegativity increases in the periodic table.
Carbon is in the 14th group to the left of the periodic table, whereas Oxygen is in the 16th group to the right. Carbon is clearly less electronegative than oxygen. Hence it should be placed at the center of the lewis diagram, with Oxygen arranged in a circle around it.
 A single bond connects Carbon and Oxygen
A single bond connects Carbon and Oxygen
In the third phase, we’ll begin drawing the Lewis structure of CO2 by using a single bond to connect the outer oxygen atom to the carbon core atom. The diagram above shows two single bonds, each containing four electrons. We drew the Lewis structure of CO2 using four of the 16 valence electrons available.
-
It has 12 valence electrons.
-
There are now only 12 valence electrons remaining.
 Put the remaining valence electrons
Put the remaining valence electrons
For Oxygen to have an octet rule, we need to first position its 12 remaining valence electrons around it. According to the Octet rule, atoms are most stable with eight electrons in their valence shell.
On the other hand, Oxygen requires 8 electrons to enter the stable zone. The remaining valence electron should be placed around Oxygen first to complete the octet shell. Take a look at the diagram above to see how many valence electrons we’ve used so far and how many we have left to use if any.
There are enough electrons in each oxygen atom to complete an octet (i.e., six dot electrons plus 2 electrons shared between two different bonds). Sixteen valence electrons are employed (6 on each oxygen atom plus 4 electrons in the form of two single bonds).
 Form a covalent bond
Form a covalent bond
The CO2 lewis dot structure has been completed, and this is the final phase. We need to complete the octet of the core atom (Carbon) to ensure its stability in this phase. It has only four electrons (two single bonds) surrounding it when it comes to Carbon, so it requires another four to complete its octet shell.
In addition, we are out of valence electrons to finish the carbon octet. As a result, Oxygen lone pair electrons will be utilized to help us solve this issue. Convert each Oxygen’s two lone pair electrons into a covalent bond.
To determine whether or not CO2 atoms have completed their eight-membered octet, examine the Lewis dot structure shown above. In fact, with 8 electrons in the outermost shell of each atom (Carbon and Oxygen), both have completed the octet rule.
 Examine the stability with a formal charge idea.
Examine the stability with a formal charge idea.
Now, using the formal charge notion, verify the stability of the CO2 Lewis structure. It has been demonstrated that the Lewis diagram is more stable when the formal charge on the atoms is lower. To figure out how much authority an atom has in standard terms.
In molecular geometry, atoms are arranged in a specific way. The lewis structure of CO2 can provide us with an approximation of its molecular shape, but the VSEPR theory is needed to determine the precise molecular geometry of CO2.
The valence electron pair repulsion (VSEPR) theory predicts the molecule’s shape by measuring the repulsion between the electron pairs. This theory can predict two distinct forms of geometry:
 The geometry of electrons
The geometry of electrons
The molecular structure of a substance To establish the form of any molecule, electron geometry considers all electrons (both bonding and antibonding). In contrast, molecular geometry only takes into account the bonding electrons.
Summary
Valence shell electron pairs around the central carbon atom and lone pair on both sides of each oxygen atom repel each other, so the repulsion on the left and right sides is the same because the central carbon atom is doubly bonded with each Oxygen.
 Lewis structure of CO2.
Lewis structure of CO2.
To grasp a molecule’s molecular geometry, one must know its Lewis structure. This structure makes understanding the arrangement of electrons in molecules and their shape easier. To grasp CO2’s Lewis structure, one must first understand what it is.
The Lewis dot structure depicts the valence shell electron configuration pictorially. The Lewis dot structure is a way to express these valence electrons by painting dots around the individual atoms. The molecule’s bonds are depicted by lines drawn on the molecule’s surface.
 The Atomic Arrangement
The Atomic Arrangement
Such a structure aids in comprehending the arrangement of atoms and the electrons that participate in the bonding process. Following our discussion of Lewis structures, let’s take a quick look at the Lewis structure of CO2.
When you look at the molecule of CO2, you’ll notice that Carbon sits right in the middle of it. The two Oxygen atoms on the terminals share electrons with the core Carbon atom and establish connections with each other. Let’s look at the valence electrons of all the particles in the molecule to see how the bonds are formed and organized.
-
Valence electrons in Carbon: 4
-
Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we will multiply it by 2)
-
Valence electrons in the molecule are totaled at 16.
Carbon should be placed in the central location, with four dots surrounding it. Place two Oxygen molecules on either side of the atom and six dots around each one to indicate the valence electrons. To achieve an electrical configuration similar to inert gases, a molecule must complete its octet to become stable and inactive.
By either contributing or absorbing an electron, this is accomplished. There are two Oxygen molecules in this system, and since Oxygen is more electronegative than Carbon, Carbon will distribute its electrons to both.
Double bonds are formed when two oxygen atoms share two electrons from the Carbon atom to complete their octets. To achieve this, each Oxygen nucleus will create a twofold connection with the central heart.
To demonstrate the presence of double bonds, draw two parallel lines connecting the Oxygen and carbon atoms. CO2 has two Oxygen atoms making double bonds with Carbon atoms in the Lewis structure. Due to all of the electrons being utilized, the molecule does not have any non-bonding pairs or lone pair electrons.
 Hybridization of carbon dioxide
Hybridization of carbon dioxide
Let’s take a quick look at CO2’s Hybridization and bond angles to understand its molecular geometry better. A Carbon atom’s ground-state electronic configuration is 1s22p2, and an Oxygen atom is 1s22p4. Jumping between orbitals occurs when electrons are stimulated.
There is now one electron in each of the p-orbitals of the atoms in the excited state, which is 1s2 2p1 2p3. 2s orbitals and one of the p orbitals will hybridize to generate two sp orbitals in this case. However, there are three hybrid orbitals in Oxygen, which hybridize to generate three electrons.
Sigma bonds are formed when the two p-orbitals of the Oxygen atom overlap with these two hybridized orbitals. In the p-orbitals of the Oxygen atom, the remaining electrons form a pi bond. CO2 has an sp hybridization because sp orbitals are hybridized to form bonds.
 Molecular Geometry of CO2
Molecular Geometry of CO2
The arrangement of atoms, electron pairs, and bonds determines any substance. Oxygen atoms in CO2 have completed their octet in two sigma bonds with the carbon atom in the molecule’s center. Bonding pairs of electrons also repel each other since there are no lone pairs of electrons.
The CO2 molecule takes on a linear shape because of these repulsion forces between the valence shell electron pairs. The result is a linear molecular geometry with 180-degree bond angles and evenly distributed electrons.
 Hypercapnia
Hypercapnia
Hypercapnia occurs when the blood contains too much carbon dioxide (CO2). As a result of not being able to adequately breathe and obtain Oxygen into your lungs, it occurs. When your body isn’t receiving enough Oxygen or exhaling enough CO2, you may need to gasp or breathe heavily to balance your Oxygen and CO2 levels.
This isn’t always a problem. For example, if you sleep deeply yet your breathing is shallow, your body reacts. You may toss and turn in your sleep. You may then breathe normally again and receive more oxygen into your blood.
| Rosy skin | |
| Sleepiness or lack of focus | |
| Headaches | |
| Dizzy or bewildered | |
| Out of breath | |
| Being excessively fatigued |
Summary
Carbon Dioxide has a linear molecular geometry, it has an sp hybridization and is 180 degrees. Asymmetric distribution of electrons in the molecule results in no lone pairs of electrons because of the repulsion between interactions.
 Frequently Asked Questions - FAQs
Frequently Asked Questions - FAQs
The most frequently asked questions concerning the Lewis dot structure for CO2 are listed here.
 What’s wrong with the Lewis dot structure for CO2?
What’s wrong with the Lewis dot structure for CO2?
It’s worth noting that C has no formal charge in the provided Lewis structure, but O has standard charges of 1 and 0. The existing lewis structure does not adequately reflect the nonpolar character of carbon dioxide. It is possible to propose a better Lewis structure.
 Is the octet rule applicable to CO2?
Is the octet rule applicable to CO2?
All four electrons in carbon dioxide are shared equally by each oxygen molecule, two of which are provided by Oxygen and two by Carbon. The octet rule applies to both atoms since all four electrons are counted in both the carbon and oxygen octets.
 Is there a reason why carbon dioxide is nonpolar?
Is there a reason why carbon dioxide is nonpolar?
The chemical formula CO2 indicates that carbon dioxide is nonpolar. It comprises two pairs of oppositely charged polar bonds… As a result, the two bond dipole moments cancel each other out, resulting in no net molecule dipole moment. Thus, the molecule has no polarity at all.
 How many bonds does CO2 have?
How many bonds does CO2 have?
Carbon dioxide is made up of two carbon atoms bonded together. One sigma bond and one bond make up a double bond. Our global carbon cycle and climate change are based on carbon dioxide as an industrial reagent.
 What kind of bonds are found in co2?
What kind of bonds are found in co2?
Each oxygen atom is linked to one of the oxygens in carbon dioxide by two covalent bonds (shown as two lines), referred to as “double bonds.” The charge distribution is uniform when symmetrical molecules because atoms pull evenly on electrons. Balanced molecules do not have polarities.
 What is the reason for CO2’s linearity?
What is the reason for CO2’s linearity?
In contrast to carbon dioxide, sulfur dioxide is bent (V-shaped). … The carbon dioxide molecule is linear because the two double bonds want to get as far apart as possible. Double bonds and the lone pair in the molecule are spaced apart to the greatest extent possible to reduce repulsions.
 Is there a triple bond in CO2?
Is there a triple bond in CO2?
Carbon (C) is the primary element in CO2. The CO2 molecule has a Tetrahedral VSEPR structure. One electron must be subtracted from the final total for each double- or triple-bonded molecule. Double bonds in CO2 mean that two electrons are removed from the final total.
 Is there a dative bond in CO2?
Is there a dative bond in CO2?
There are four carbon-oxygen atoms in carbon dioxide. Because each atom donates an electron to form a bond, option (B) is erroneous because Carbon dioxide does not include a dative bond. Two electrons from each nitrogen and Oxygen are shared to form two bonds in nitric oxide.
 In what ways does CO2 undergo Hybridization?
In what ways does CO2 undergo Hybridization?
Sp hybridization is the most common type of carbon dioxide Hybridization. Because Carbon is bonded to two other atoms, this Hybridization occurs. Bonds can be two double bonds or one single bond and a triple bond.
 The octet of CO2 is complete?
The octet of CO2 is complete?
As illustrated in the diagram below, carbon dioxide contains one carbon atom and two oxygen atoms. In comparison to Carbon, each Oxygen atom possesses six valence electron . Carbon needs four extra valence electrons to meet the Octet Rule.
Conclusion
Two electrons are now present in each of the p-orbitals of the atoms in the excited state (1s2 2p1), and one of the p orbitals will hybridize with one of the 2s orbitals to form two sp orbitals in this exam le. On the other hand, Oxygen has three hybrid orbitals that combine to produce three electrons.
A periodic table can be used to determine the valence electrons of Carbon and Oxy en. Carbon is found in 14 groups on the periodic table, while Oxygen is found in 16. Unlike Carbon, Oxygen has six valence electrons instead of four.
 Related Articles
Related Articles
2 - Liquid carbon