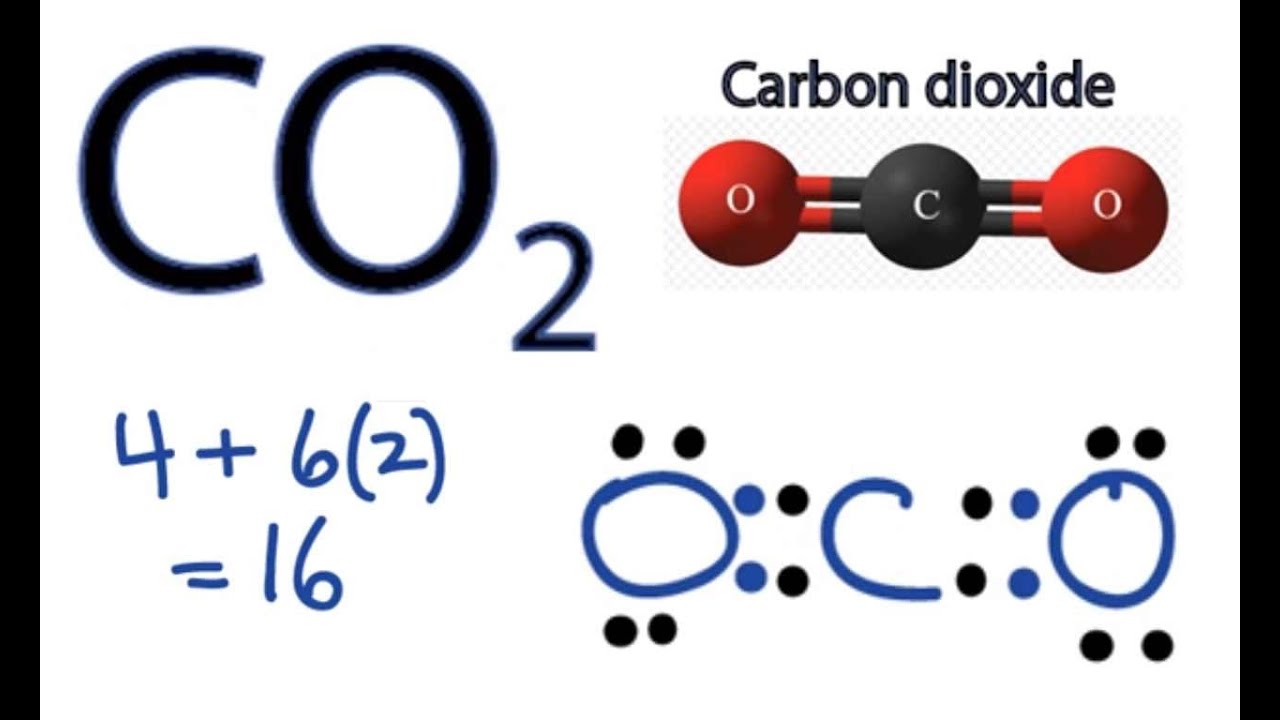Lewis dot diagram for CO2 features two double bonds traveling from carbon to the oxygen atoms. According to the octet rule, each oxygen atom has to bind twice and the carbon atom needs to bond four times.
About Carbon Dioxide (CO2)
When first learning about Lewis dot diagram and Molecular Geometry, a good place to start is with Carbon Dioxide. First-time students who wish to understand the principles of Lewis dot structures and how to draw them should start with this molecule.
There are two atoms in CO2 or Carbon Dioxide: carbon and oxygen. Although this gaseous molecule is well-known for its role in the greenhouse effect and global warming, there are several applications for this gas in a variety of sectors.
The molecular geometry of a substance is critical to understanding its physical qualities, reactivity, and other chemical aspects. I’ve included some information on the Lewis dot diagram for CO2 and hybridization below to aid your comprehension.
| Name of Molecules | Carbon Dioxide (CO2) |
|---|---|
| Valence Electron | 16 |
| Hybridization | Sp hybridization |
| Bond Angles | 180 degrees |
| Molecular Geometry | Linear |
CO2 Lewis Dot Diagram
Any molecule’s molecular geometry cannot be understood without first having a firm grasp of the Lewis structure. To better understand the arrangement of electrons in molecules and their form, this structure is useful. Before learning about the Lewis dot diagram for CO2, it is necessary to have a firm grasp of what constitutes a Lewis structure.
The valence shell electron configuration of a molecule is shown pictorially by the Lewis dot structure. As a result of the Lewis dot structure, the valence electrons may be seen by placing small dots around the atoms. The molecule’s bonds are shown by lines drawn on the surface of the molecule.
Understanding the arrangement of atoms and electrons involved in the bond formation is made easier by this structure. Here is the Lewis dot diagram for CO2 to show you how it is drawn and how it is used.
The Carbon atom has the most prominent position in CO2 because it is the molecule’s least electronegative atom. Carbon atoms share electrons with the two Oxygen atoms on the terminals and link to each other.
Let’s look at the valence electrons of all the atoms in the molecule to see how the bonds are formed and how they are organized.
Carbon Valence Electron: 04
Oxygen valence electrons: 6*2 = 12
Total valence electron: 16
So, for the time being, put Carbon in the middle and circle it with four dots. In addition to the location, two Oxygen atoms on each side of the atom, and six dots surrounding each atom symbolize the valence electrons.
A molecule may become stable and inactive by attaining an electrical state comparable to that of inert gases by completing its octet. This may be accomplished in one of two ways: by giving or receiving an electron. In this case, Carbon will transfer its electrons to both of these Oxygen atoms since Oxygen is more electronegative than Carbon.
Since the octets of two oxygen atoms each need two electrons, the two electrons from the carbon atom are shared to create double bonds. A double bond is therefore formed between each Oxygen atom and the center atom.
Double bonds between Oxygen and Carbon atoms may now be shown by drawing two parallel lines connecting them. In the Lewis dot diagram for CO2, two Oxygen atoms will now form double bonds with a Carbon atom, completing the Lewis structure.
There are no lone pairs of electrons or non-bonding pairs of electrons in the molecule since all the valence electrons of all the atoms are utilized.
A fast review of CO2’s hybridization and bond angles will help us better comprehend the molecular geometry of the gas, so let’s get started.
CO2 Hybridization
Carbon has an electrical configuration of 1s2 2s2 2p2 in its ground state, while Oxygen has 1s2 2s2 2p4 in its ground state. Electrons may hop to other orbitals when they’re energized.
The atom’s electrical configuration changes to 1s2 2s1 2p3 in its excited state, resulting in one electron in each of the atom’s p-orbitals. 2s orbitals and one p-orbital will hybridize to generate 2 sp orbitals in this example. There are three hybrid orbitals in Oxygen, however, which hybridize to generate three electrons.
The two p-orbitals of the Oxygen atom overlap with these two hybridized orbitals, resulting in the creation of sigma bonds. Pi bonds are formed by the Oxygen atom’s remaining electrons in the p-orbitals. CO2 has an sp hybridization because sp orbitals are hybridized to create the bonds.
CO2 Molecular Geometry
The arrangement of atoms, electron pairs, and bonds determines the molecular geometry of each substance. As in CO2, the central carbon atom forms sigma bonds with both Oxygen atoms, thereby completing the octet chain. Because there are no lone pairs of electrons, bonding pairs of electrons also repel each other.
The CO2 molecule takes on a linear form to minimize the repulsion between the electron pairs in the valence shell. Consequently, CO2 has a linear molecule geometry with 180-degree bond angles and symmetric electron distribution.
Outline:
To sum up, the Molecular Geometry of Carbon Dioxide is Linear. It features a 180-degree bond angle and sp hybridization. The electrons in the molecule are distributed symmetrically, therefore there are no lone pairs. CO2 has a linear shape because of the repulsion forces between the pairs of electrons.
How to Draw the Lewis Dot Diagram for CO2?
The lewis dot diagram for CO2 may be drawn in several ways, and if you study this well, you’ll be able to understand:
-
For carbon and oxygen, calculate the total number of electrons in their valance shells.
-
The total number of electron pairs, including lone pairs and bonds, that occur in nature.
-
Determine the atom’s center of gravity and sketch it out.
-
Identify atoms with lone pairs by marking them.
-
If there are charges on atoms, note them in the appropriate places on the diagram.
-
The optimum lewis structure may be achieved by converting lone pairs to bonds and minimizing the number of atoms with negative charges.
Electrons in CO2 Valence Shells
Carbon dioxide is composed of oxygen and carbon. Oxygen has six electrons in its final shell, making it a member of group VIA. Carbon is a member of the IVA group and possesses four valence electrons. We now know how many electrons are contained inside the oxygen atom’s valence shells.
The number of electrons in the valance shell multiplied by the number of atoms in the element will give you the total number of valence electrons provided by that element.
Carbon Valence Electron: 04
Oxygen valence electrons: 6*2 = 12
Total valence electron: 16
Ozone’s Overall Valence Electron Pair
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
The number of total valence electrons is divided by two to get the total number of electron pairs. The number of electrons in CO2 is 8.
The CO2 molecule’s central atom is sketched and chosen. Because carbon’s valence (4) is greater than oxygen’s, we know it has the best probability of serving as the carbon dioxide’s center atom (2).
Lone Pairs on Atoms
-
We may begin marking lone pairs on atoms after establishing the core atom and drawing the CO2 molecule. Keep in mind that the total number of electron pairs is eight.
-
Because of this, there are only six pairs of electrons left to identify atoms.
-
Most of the time those leftover pairs of paired electrons should be used to indicate outwardly. The oxygen atom can only hold eight electrons in its last shell, so keep that in mind as well! As a result of these characteristics, oxygen atoms may be marked with electron pairs.
-
During this stage, each oxygen will maintain three separate pairings. The last six pairs of electrons have already been identified.
-
In carbon, there are no more lone pairs to indicate.
-
Draw the Lewis dot diagram for CO2 by identifying the lone pairs.
-
Check the octal rule and atom charges
It is important to note any charges on the atoms shown in the above CO2 structure. Each oxygen and carbon atom does have charges, as seen in the following image. It is critical to know the charges to arrive at the finest Lewis structure possible. The reason we’re looking for charges is because of this.
Analyze the Stability of Atoms and Reduce their Electrical Charges by Joining Lone Pairs
A molecule’s structure is unstable if each atom has a charge. As a result, we must minimize atom-charge densities. The initial stage in lowering charges is to form a connection between a lone pair of oxygen atoms and a carbon atom.
Charges on atoms have been lowered. If possible, we should also lower fees. Yes, it is possible to join a single oxygen atom to a carbon atom by converting it to a bond. Then, all atoms will be free of charge. There are two double bonds surrounding carbon atoms in the lewis dot diagram for CO2.
Summary:
The Lewis dot structure is made up of letters that represent the element’s atoms, surrounded by dots or dashes. Dots indicate shared electrons within atom bonds, whereas dashes represent covalent links. Instead of the single lines that normally connect atoms in a molecule, lone pairs of electrons are displayed as dots encircling atoms.
Frequently Asked Questions
Here are some FAQs related to the Lewis dot diagram for CO2:
1. What’s wrong with the CO2 molecule’s Lewis dot structure?
The C atom has a formal charge of 0 in the given lewis structure, whereas the O atoms have a formal charge of +1 and -1. As a result, the existing lewis structure does not represent the non-polar nature of CO2. It is possible to suggest a better Lewis structure.
2. What are the Lewis dot diagram’s drawbacks?
During the formation of a covalent bond, energy is released. Molecule shapes could not be clarified using this technology. The energy is emitted when covalent bonds are formed. Atomic interactions in molecules are characterized by their attractive forces.
3. What is the Lewis dot structure for carbon dioxide?
Each valence electron in carbon is used to make four bonds. As a result, four little dots surround the element carbon. The two lone dots to the left and right of the O atoms illustrate the only links oxygen requires. Dots above and below the Os will not form a solid connection.
4. Is CO2 a full octet of atoms?
There is one carbon atom in carbon dioxide, and there are two oxygen atoms in carbon dioxide. In contrast to Carbon, each Oxygen atom possesses six valence electrons. Carbon requires four extra valence electrons to meet the Octet Rule.
5. If so, what is the rule for CO2?
For the octet or eight electrons, the valence electrons of carbon and oxygen must be shared, as each holds six electrons in its valence shells. As a result, the octet rule applies to carbon dioxide.
6. In what ways is the Lewis model superior to other models?
Since there are so many acids and so many acid-base reactions, Lewis’ hypothesis has a major benefit. If an acid can receive two valence electrons, it is an acid, according to the Lewis hypothesis.
7. What gives rise to the inaccuracy of Lewis’ structures?
Electrons pulled into the structure When building a Lewis Structure, make sure that the number of electrons is equivalent to the number of valence electrons in the substance being used. The diagram is wrong if the number of electrons in the figure does not match the number of valence electrons.
8. Carbon dioxide should have how many electrons?
Adding 8 electrons to the valence shells of the CO2 molecule, there are two oxygen atoms each contributing two electrons. Because carbon is neutrally charged, no more electrons are required, resulting in a final electron count of 8.
9. Carbon dioxide is nonpolar for what reason?
A polar covalent bond is one in which two atoms with differing electronegativities share electrons unequally. These two polar bonds are found in both CO2 and H2O. As a result, CO2 molecules are non-polar because the dipoles in the CO2 molecule cancel one another.
10. In what way does carbon dioxide bind?
Carbon dioxide CO2 is formed when one carbon atom joins two oxygen atoms. The strong C=O bond in the carbon dioxide molecule keeps it together. electrons are shared in carbon-oxygen double covalent bonds
Conclusion
In the Lewis dot diagram for carbon dioxide (CO2), two oxygen atoms are joined by a carbon atom. CO2 has two carbon atoms surrounded by double bonds. While there are no single lone pairs on carbon atoms, there are two single lone pairs on the valence shells of each oxygen atom. CO2 has a linear shape. This page explains in detail how to sketch CO2’s lewis dot diagram.
Related Articles
Lewis dot structure for CO2
Lewis dot structure for Ch2cl2