Is carbon dioxide a pure substance? Yes, carbon dioxide is a pure substance, not a mixture. Carbon dioxide is a pure substance since its composition remains constant. Each molecule will always have one carbon and two oxygens. Carbon dioxide is a pure substance because it has a fixed texture no matter where it was taken. Each molecule of carbon dioxide will always have 1 carbon and 2 oxygen.
Carbon dioxide
Carbon dioxide shows up as a lackluster unscented gas at climatic temperatures and tensions. Somewhat nontoxic and noncombustible. Heavier than air and may suffocate by the removal of air.
-
Dissolvable in water. Structures carbonic corrosive, a gentle corrosive. Under delayed openness, to hotness or fire, the compartment might crack savagely and rocket.
-
Used to freeze food, to control compound responses, and as a fire quenching specialist.
-
Carbon dioxide is a one-carbon compound with recipe CO2 in which the carbon is joined to every oxygen particle by a twofold bond.
-
A drab, unscented gas under ordinary conditions, it is delivered during breath by all creatures, organisms, and microorganisms that rely straightforwardly on or by implication upon living or rotting plants for food.
-
It plays a part as a dissolvable, a vasodilator specialist, a sedative, the main enemy, an individual from ozone-depleting substance, a human metabolite, an individual from food bundling gas, a food charge, a refrigerant, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite.
-
It is a one-carbon compound, a gas atomic element, and carbon oxide.
-
Carbon Dioxide is a boring, scentless, incombustible gas coming about because of the oxidation of carbon.
Carbon dioxide is an acidic boring gas with a thickness around 53% higher than that of dry air. Carbon dioxide particles comprise of a carbon molecule covalently twofold attached to two oxygen iotas. It happens normally in Earth’s air as a follow gas.
|Molar mass|44.01 g/mol|
| — | — |
|Formula|CO2|
|Boiling point|-78.46 °C|
|Triple point temperature|-56.6 °C|
|ChemSpider ID|274|
|Soluble in|Water|
|Classification|Acidic oxide|
Summary
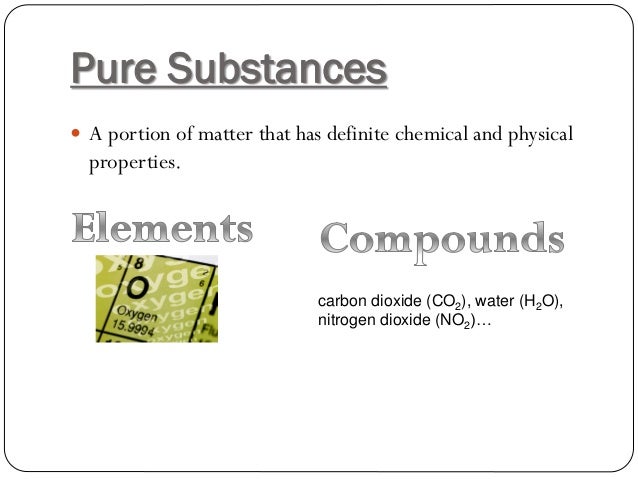
Carbon dioxide is an unadulterated substance. An unadulterated substance should be made out of only one kind of particle, or it very well might be anionic or metallic strong made solely out of the molecules in the experimental equation, albeit not generally of a similar sort of iota.
CO2 Atomic Calculation
Carbon dioxide is a gas with a set-piece that holds its personality in any event, when exposed to minor changes in actual conditions, for example, temperature and strain and needs compound alterations to change into different substances.
To respond to the inquiry CO2 is polar or nonpolar, we want to comprehend the sub-atomic calculation of CO2. In CO2 sub-atomic math, Carbon makes a twofold bond with every one of the two oxygen particles, bringing about a minuscule even, the straight particle of CO2 that is unpredictable and sensibly receptive.
-
Since oxygen iotas make sigma bonds with the focal carbon particle to finish their octet, CO2 has a straight atom shape.
-
Thus, solitary sets of electrons are missing, yet reinforced sets of electrons repulse each other.
-
Because of these loathsome powers between the valence shell electron matches, the particle secures a direct shape.
Properties of carbon dioxide an unadulterated substance
First, think about the properties of an unadulterated substance:
-
Made of just one kind of particle or atom
-
Has a distinct softening point, limit, shading, scent, thickness, and solvency.
-
Instances of unadulterated substances incorporate components like iron, silver, mercury, and so forth What’s more mixtures like water, carbon dioxide, methane, vinegar.
Presently think about the properties of a combination :
-
Materials don’t artificially consolidate
-
Shifting physical and substance properties
-
Instances of combinations incorporate mechanical blends like soil, arrangement combinations like sugar in espresso, suspension blends like tomato juice, and colloid combinations like milk or ketchup.
Is carbon dioxide component compound or component?
Carbon dioxide is a compound as it is made out of more than one component. It has one carbon particle and two oxygen molecules
Sub-atomic math
Direct
CO2 has 2 electron areas, bringing about a straight electron space calculation. Both electron areas are holding sets, so CO2 has straight sub-atomic math with a bond point of 180°.
Intermolecular powers
CO has two C-O bonds. The dipoles point in inverse ways, so they counteract one another. Along these lines, even though CO₂ has polar bonds, it is a nonpolar particle. Thusly, the main intermolecular powers are London scattering powers.
Valence electrons
8 electrons
For the CO2 particle, there are 2 oxygen molecules which contribute 2 electrons each, so adding the 4 electrons to the valance shells sums 8 electrons. The carbon has no charge, so no additional electrons are required so the last absolute is 8.
Outline Practice issues
Carbon dioxide is an acidic drab gas with a thickness around 53% higher than that of dry air. Carbon dioxide particles comprise a carbon molecule covalently twofold clung to two oxygen iotas. It happens normally in Earth’s air as a follow gas.
CO2 has 2 electron spaces, bringing about a direct electron area calculation. Both electron areas are holding sets, so CO2 has a straight atomic calculation with a bond point of 180°
-
co2 atomic calculation
-
Intermolecular powers
CO has two C-O bonds. The dipoles point in inverse ways, so they counterbalance one another. Accordingly, even though CO₂ has polar bonds, it is a nonpolar atom.
What sort of intermolecular power of fascination is found in co2? | Socratic
-
co2 intermolecular powers
-
Valence electrons
-
8 electrons
For the CO2 particle there are 2 oxygen molecules which contribute 2 electrons each, so adding the 4 electrons to the valance shells aggregates 8 electrons. The carbon has no charge, so no additional electrons are required so the last absolute is 8.
Thickness of Carbon is 1562 kg/m3 (strong at 1 atm (100 kPa) and 78.5 °C (−109.3 °F)) 1101 kg/m3 (fluid at immersion −37 °C (−35 °F)) 1.977 kg/m3 (gas at 1 atm (100 kPa) and 0 °C (32 °F)
Is Carbon Dioxide a Blend?
No, carbon dioxide isn’t a blend. Carbon dioxide is made out of carbon synthetically clung to oxygen. As a result of this compound bond that is hard to break, carbon dioxide isn’t viewed as a combination.
-
All things being equal, carbon dioxide is viewed as a compound.
-
Feeling befuddled? Allow us to clarify, and answer some more carbon dioxide-related inquiries.
Why Is Carbon Dioxide a Blend?
As we referenced in the introduction, the components that makeup carbon dioxide (carbon and oxygen) are synthetically reinforced. They don’t fall apart effectively by any means.
-
A combination is a material where the substances in the blend are not synthetically clung to one another.
-
Subsequently CO2 doesn’t qualify.
-
All things being equal, carbon dioxide is all the more accurately called a compound, which is a substance where the parts are artificially clung to one another.
Is Carbon Dioxide Homogeneous?
Assuming you are discussing unadulterated carbon dioxide gas, then, at that point, indeed, CO2 is homogeneous.
-
To be homogeneous, a substance should be synthetically steady all through its parts. Assuming that you test it in one spot, the example will be the equivalent artificially as an example taken from somewhere else.
-
Since carbon dioxide is a gas, the atoms are bouncing all around out of control in whatever contains the gas is contained in.
-
There is no assurance that you’ll get a similar number of CO2 atoms in a single example as you would get in another.
-
Be that as it may, with unadulterated CO2, you will not be getting some other components, mixtures, or blends all things considered. To this end, we say that it is homogeneous.
Summary
Unadulterated oxygen gas comprises of atoms yet it is as yet thought to be a component, rather than a compound, as the particles are comprised of a solitary sort of component. Compounds are comprised of at least one component.
Unadulterated Substances
At the point when we talk about an unadulterated substance, we are discussing something that contains just one sort of issue.
This can either be one single component or one single compound, yet every example of this substance that you look at should contain the very same thing with a fixed, positive arrangement of properties
Combinations
Assuming that we take at least two unadulterated substances and combine them as one, we allude to this as a combination. Blends can forever be isolated again into part unadulterated substances since holding among the iotas of the constituent substances doesn’t happen in a combination.
-
While a compound might have altogether different properties from the components that create it, it blends the substances to keep their singular properties.
-
For instance sodium is a delicate sparkly metal and chlorine is a sharp green gas. These two components can consolidate to frame the compound, sodium chloride (table salt) which is a white, translucent strong having none of the properties of one or the other sodium or chlorine.
-
Assuming, be that as it may, you blended table salt in with ground pepper, you would, in any case, have the option to see the singular grains of every one of them and, assuming you showed restraint, you could take tweezers and cautiously separate them back into unadulterated salt and unadulterated pepper.
Heterogeneous combination
A heterogeneous blend is a combination where the sythesis isn’t uniform all through the blend. Vegetable soup is a heterogeneous blend. Some random spoonfuls of soup will contain fluctuating measures of the various vegetables and different parts of the soup.
Homogeneous blend/Arrangement
A homogeneous blend is a mix of at least two substances that are personally blended that the combination acts as a solitary substance. One more word for a homogeneous combination is the arrangement.
-
Hence, a blend of salt and steel fleece is a heterogeneous combination since it is not difficult to see which particles of the matter are salt precious stones and which are steel fleece.
-
Then again, assuming you take salt precious stones and break down them in water, it is undeniably challenging to tell that you have more than one substance present just by looking regardless of whether you utilize an amazing magnifying instrument.
-
The salt disintegrated in water is a homogeneous combination or an answer.
Arranging Matter
Connections between the Kinds of Issue and the Strategies Used to Isolate Blends
Common table salt is called sodium chloride. It is viewed as a substance since it has a uniform and unmistakable creation. All examples of sodium chloride are synthetically indistinguishable. Water is likewise an unadulterated substance.
-
Salt effectively breaks down in the water, however, saltwater can’t be delegated a substance since its piece can fluctuate. You might break down a modest quantity of salt or a huge sum into a given measure of water.
-
A combination is an actual mix of at least two parts, every one of which holds its personality and properties in the blend.
-
Just the type of the salt is changed when it is disintegrated into water. It holds its synthesis and properties.
Stage
A stage is any important example that has a uniform structure and properties. By definition, an unadulterated substance or a homogeneous blend comprises a solitary stage.
A heterogeneous blend comprises at least two stages. At the point when oil and water are consolidated, they don’t blend equally, yet rather structure two separate layers. Every one of the layers is known as a stage.
Summary
Matter can be ordered into two general classifications: unadulterated substances and combinations. An unadulterated substance is a type of issue that has a consistent piece and properties that are steady all through the example. Blends are actual mixes of at least two components as well as mixtures.
Important Key Factors
Unadulterated substances are made out of a solitary component or Blends can be named homogeneous or heterogeneous. Components and mixtures are the two instances of unadulterated substances. Compounds are substances that are comprised of more. Blends can be named homogeneous or heterogeneous. Components and mixtures are the two instances of unadulterated substances. Compounds are substances that are comprised of more.
-
Blends of various substances are called combinations.
-
Homogeneous blends are combinations of at least two mixtures (or components) that are not outwardly recognizable from one another.
-
Heterogeneous blends are combinations of at least two mixtures (or components) that are outwardly discernable from each other.
Is Carbon Dioxide Pure Substance?
Carbon dioxide is a compound that comprises of two molecules of oxygen and an iota of carbon. Unadulterated substances don’t need to be blended and can’t be separated without complex cycles.
Since carbon dioxide is regular and can’t be separated effectively, it’s an unadulterated substance.
The Air We Relax
We take in oxygen from the air when we inhale, and we additionally discharge carbon dioxide. The air we inhale is comprised of a few substances. We will quite often consider oxygen being the fundamental part of air because our bodies need it to live, however, oxygen is only a little piece of the cosmetics of air. All things considered, 78% of the air that we inhale is nitrogen, while another 21% is oxygen.
-
Different gases make up what’s left of the synthetic creation of air. Argon, methane, helium, krypton, hydrogen, xenon, and iodine are available in modest quantities.
-
You can track down modest quantities of mixtures in the air too Carbon dioxide is in the air we inhale, alongside ozone, nitrogen dioxide, and a follow measure of carbon monoxide. There are likewise limited quantities of water fume in the air we relax.
The Trading of Oxygen and Carbon Dioxide
At the point when we inhale, we’re getting the oxygen we want from the air to get by. The cells in our body use oxygen to make energy. As we bring air into our lungs, a huge number of small sacs called alveoli to take the oxygen in.
-
The dividers of the alveoli are adequately slim to deliver the Blends can be named homogeneous or heterogeneous. Components and mixtures are the two instances of unadulterated substances. Compounds are substances that are comprised of moreinto our circulation system, and our blood conveys oxygen all through our body.
-
The energy that our bodies exhaust makes carbon dioxide, so the breathing system works backward to eliminate the carbon dioxide from our bodies.
-
Our blood conveys carbon dioxide back to the lungs, where we discharge it as we breathe out.
How Plants and Trees Help Us With soothing
Plants and trees assist us with the trade between carbon dioxide and oxygen during the day by doing the opposite of what people do. Plants don’t actually inhale as we do in light of the fact that they don’t have lungs, yet what they do is an interaction called photosynthesis and breath.
-
Around evening time, plants take in oxygen and delivery Blends can be named homogeneous or heterogeneous. Components and mixtures are the two instances of unadulterated substances. Compounds are substances that are comprised of moredioxide very much as we do. During the day, plants utilize the light of the sun to take in carbon dioxide to make supplements.
-
They discharge oxygen back into the air. This cycle is the reason plants are so significant for human endurance since they produce such a great deal the oxygen we really want to make due.
Carbon Dioxide and Contamination
Despite the fact that carbon dioxide happens normally and is a significant piece of the existence of plants, there’s an unmistakable connection between carbon dioxide and contamination.
-
A significant part of the contamination in our climate happens as a result of an excessive amount of carbon dioxide that industrial facilities and petroleum product consuming transportation makes.
-
Advocates of environmental change accept that Blends can be named homogeneous or heterogeneous. Components and mixtures are the two instances of unadulterated substances. Compounds are substances that are comprised of more with carbon dioxide from the air will forestall warming of the planet.
-
Hippies accept that changing the fills we use will assist cut with bringing down on the carbon dioxide that we discharge into the air.
Different Types of Carbon Dioxide
Carbon dioxide is a gas, and we can’t see or taste it when we inhale air. In any case, we can observer carbon dioxide in different structures.
-
The bubble in soda pops, shimmering wine, and a few Blends can be named homogeneous or heterogeneous. Components and mixtures are the two instances of unadulterated substances. Compounds are substances that are comprised of more of brew comes from carbon dioxide. The air pockets and bubble are the impacts of the carbon dioxide as it’s delivered once again very high, and it’s the reason we allude to these sort of beverages as carbonated drinks.
-
In the event that you’ve seen dry ice, you’ve seen one more type of carbon dioxide.
-
Dry ice is frozen carbon dioxide, and it’s cool that the air that hits it consolidates and makes mist, which is the reason we see it used to make mist and cloudiness for plays and shows.
-
At the coldest temperature ever in Antarctica in 1983, carbon dioxide transformed into snow, and space researchers have made a note of carbon dioxide snow on Mars too.
Substances and Combinations
Important keypoint
Matter can be separated into two classifications: unadulterated substances and blends. Unadulterated substances are additionally separated into components and mixtures. Combinations are genuinely consolidated constructions that can be isolated into their unique parts.
-
A synthetic substance is made out of one sort of particle or atom.
-
A combination is made out of various sorts of particles or atoms that are not artificially reinforced.
-
A heterogeneous blend is a combination of at least two compound substances where the different parts can be outwardly recognized.
-
A homogeneous blend is a sort of combination wherein the organization is uniform and all aspects of the arrangement has similar properties.
-
Different partition methods exist to isolate matter, including incorporate refining, filtration, vanishing and chromatography. Matter can be in a similar stage or in two distinct stages for this detachment to occur.
Terms
SubstanceA type of issue that has consistent compound piece and trademark properties. It is made out of one sort of particle or atom.
-
elementA compound substance that is comprised of a specific sort of molecule and can’t be separated or changed by a synthetic response.
-
mixtureSomething that comprises of different, non-fortified components or atoms.
Synthetic Substances
In science, a synthetic substance is a type of issue that has steady compound arrangement and trademark properties. It can’t be isolated into parts without breaking substance bonds. Compound substances can be solids, fluids, gases, or plasma. Changes in temperature or tension can make substances shift between the various periods of issue.
-
A component is a synthetic substance that is comprised of a specific sort of molecule and henceforth can’t be separated or changed by a compound response into an alternate component.
-
All particles of a component have similar number of protons, however they might have various quantities of neutrons and electrons.
-
An unadulterated synthetic compound is a synthetic substance that is made out of a specific arrangement of atoms or particles that are synthetically reinforced.
-
At least two components joined into one substance through a synthetic response, like water, structure a substance compound. All mixtures are substances, yet not all substances are compounds.
-
A synthetic compound can be either particles reinforced together in atoms or gems in which iotas, particles or particles structure a translucent grid. Compounds made principally of carbon and hydrogen molecules are called natural mixtures, and all others are called inorganic mixtures.
-
Compounds containing connections among carbon and a metal are called ogan metallic compounds.
-
Synthetic substances are regularly called ‘unadulterated’ to separate them from combinations. A typical illustration of a synthetic substance is unadulterated water; it generally has similar properties and a similar proportion of hydrogen to oxygen whether it is disengaged from a waterway or made in a research center.
-
Other synthetic substances usually experienced in unadulterated structure are jewel (carbon), gold, table salt (sodium chloride), and refined sugar (sucrose).
-
Basic or apparently unadulterated substances found in nature can truth be told be combinations of synthetic substances.
-
For instance, faucet water might contain limited quantities of disintegrated sodium chloride and mixtures containing iron, calcium, and numerous other synthetic substances. Unadulterated refined water is a substance, yet seawater, since it contains particles and complex atoms, is a blend.
Substance Blends
A blend is a material framework comprised of at least two distinct substances, which are blended however not joined artificially.
-
A blend alludes to the actual mix of at least two substances in which the personalities of the singular substances are held. Blends appear as combinations, arrangements, suspensions, and colloids.
-
Normally happening sulfur crystalsSulfur happens normally as essential sulfur, sulfide, and sulfate minerals and in hydrogen sulfide. This mineral store is made out of a combination of substances.
Heterogeneous Combinations
A heterogeneous combination is a combination of at least two synthetic substances (components or mixtures), where the various parts can be outwardly recognized and effectively isolated by actual means. Models include:
-
Combinations of sand and water
-
Combinations of sand and iron filings
-
A combination rock
-
Water and oil
-
A plate of mixed greens
-
Trail blend
-
Combinations of gold powder and silver powder
Intelligent
Oil and Water Explore the collaborations that prompt water and oil to isolate from a combination.
Homogenous Blends
A homogeneous blend is a combination of at least two synthetic substances (components or mixtures), where the various parts can’t be outwardly recognized. The structure of homogeneous blends is consistent.
-
Frequently isolating the parts of a homogeneous blend is more difficult than isolating the parts of a heterogeneous combination.
-
Recognizing homogeneous and heterogeneous combinations involves the size of testing.
-
On a little enough scale, any combination can be supposed to be heterogeneous, on the grounds that an example could be just about as little as a solitary atom.
-
In pragmatic terms, on the off chance that the property of interest is the equivalent paying little heed to the amount of the blend is taken, the combination is homogeneous.
-
A combination’s actual properties, like its dissolving point, may vary from those of its singular parts. A few blends can be isolated into their parts by physical (mechanical or warm) implies.
Is Carbon Dioxide An Unadulterated Substance Homogeneous Or Heterogeneous?
Carbon dioxide is a substance compound made out of one carbon and two oxygen molecules
Is honey a homogeneous blend?
Homogeneous blends: This sort of combinations has uniform appearance and piece all through. Presently, since honey is a combination of different sorts of sugar mixtures and it has indistinguishable properties all through and can’t be isolated into its parts. Along these lines, you can say that honey is a homogeneous combination.
Is organic product salad a homogeneous combination?
For instance, an organic product salad is a heterogeneous blend. … A homogeneous combination is a blend that is all around very much blended. It’s extremely very much blended that you can’t see the various pieces of the combination. Everything looks consistent.
Will an unadulterated substance be homogeneous?
In the more broad sense, an unadulterated substance is any homogeneous combination. That is, it is matter that seems uniform by all accounts and arrangement, regardless of how little the example size.
Instances of unadulterated substances incorporate iron, steel, and water. Air is a homogeneous combination that is regularly viewed as an unadulterated substance.
Is tea a homogeneous combination?
A Tea is an answer of mixtures in water, so it isn’t artificially unadulterated. B In light of the fact that the arrangement of the arrangement is uniform all through, it is a homogeneous blend.
What are examples of homogeneous mixtures?
Here are some examples of homogeneous mixtures:
|No. 1|Sea|
| — | — |
|No.2|Water|
|No.3|Wine|
|No.4|Vinegar|
|No.5|Steel|
|No.6|Brass|
|No.7|Air|
|No.8|Natural gas|
|No.9|Blood|
Is the human body homogeneous or heterogeneous?
In the human body, blood plasma is an illustration of a homogeneous blend. The dreary liquid holds platelets in suspension. It makes up somewhat more than half of the volume of human blood. Milk is a homogeneous colloid.
What are instances of heterogeneous blends?
Instances of Heterogeneous MixturesConcrete is a heterogeneous combination of a total: concrete, and water.Sugar and sand structure a heterogeneous blend. Ice 3D shapes in cola structure a heterogeneous combination. Salt and pepper structure a heterogeneous mixture.Chocolate chip treats are a heterogeneous blend.
Is pizza a homogeneous blend?
In a homogeneous combination the parts are consistently circulated all through the blend. Homogeneous combinations are arrangements, like salt water. In a heterogeneous blend, the parts are not consistently conveyed, like rock, or pizza.
Is carbon an unadulterated substance or blend?
Components can NOT be isolated into different sorts of issue (truly or artificially). Accordingly, components are unadulterated substances. Ex: Hydrogen gas is an unadulterated substance, as it is comprised of just Hydrogen particles or atoms. Carbon, gold, and nitrogen are different components (unadulterated substances).
Is salt water a homogeneous combination?
Homogeneous Combinations A homogeneous blend is a blend wherein the sythesis is uniform all through the combination. The salt water portrayed above is homogeneous in light of the fact that the broke down salt is equally dispersed all through the whole salt water test.
Is squeezed apple a homogeneous blend?
Despite the fact that there are a few distinct mixtures and some of them aren’t really broken up in the fluid, since you can’t really recognize them utilizing just your eyes and they truly do no different normally it is really a homogeneous combination. On the grounds that in squeezed apple there has no some other combination .
Is saline arrangement heterogeneous or homogeneous?
Instances of homogeneous combinations incorporate air, saline arrangement, most amalgams, and bitumen. Instances of heterogeneous combinations incorporate sand, oil and water, and chicken noodle soup.
What are 10 instances of heterogeneous combinations?
The following are 10 instances of heterogeneous mixtures:Cereal in milk is an extraordinary illustration of a heterogeneous mixture.Oil and water structure a heterogeneous mixture.Orange juice with mash is a heterogeneous combination. Sandy water is a Electron dot structures of co2 combination. A pepperoni pizza is a heterogeneous combination.
Is Carbon Dioxide an Unadulterated Substance?
Indeed, carbon dioxide is an unadulterated substance.
-
An unadulterated substance is one that contains just one sort of issue. An Electron dot structures of co2 substance could be a solitary component (like Gold), or it very well may be a compound (like water).
-
Each example of this substance should be actually something very similar. It acts something very similar, and responds something very similar.
-
Since carbon dioxide will likewise have a similar creation regardless of where the equivalent is taken from, and it will act something similar for each situation, carbon dioxide is an unadulterated substance.
Is Carbon Dioxide a Component?
No, carbon dioxide isn’t a component. A straightforward method for thinking about a component is to consider it a substance which can’t be separated into any easier parts. Carbon dioxide is comprised of carbon clung to oxygen.
-
The carbon and oxygen can be fallen to pieces, into independent carbon and oxygen materials.
-
To this end carbon dioxide isn’t a component.
Frequently Ask Questions
Here, some question described related to this article .
1. Is Carbon Dioxide an Ozone harming substance?
Indeed, carbon dioxide is viewed as an ozone harming substance. Indeed, it is believed to be the essential issue gas of all (which incorporates methane, nitrous oxide, and fluorinated gases).
2. Is dry ice carbon dioxide?
Indeed, dry ice is carbon dioxide. Truth be told, it is carbon dioxide that has been frozen strong. The haze you see is a combination of carbon dioxide gas and cold damp air, which is framed as the dry ice softens.
3.Is Carbon Dioxide Hydrophobic?
Indeed, carbon dioxide is viewed as a hydrophobic gas.
A hydrophobic gas is one that will in general repulse water, or neglect to blend in with it.
4. Is Carbon Dioxide Inorganic? (Or then again Natural?)
Carbon dioxide is delegated an inorganic compound. As a rule, to be viewed as natural, the material should contain some carbon to hydrogen bonds.
CO2 has no hydrogen bonds to its carbon.
As a general rule, most carbon compounds (like carbides, carbonates, and cyanides) are viewed as inorganic.
5. Is Carbon Dioxide Combustible?
By and large, CO2 is certainly not a combustible gas (like oxygen). Indeed, it isn’t unexpected utilized in fire dousers, and can assist put with trip fires.
All things considered, its peril lies keeping you from taking in sufficient oxygen.
You can take in CO2 very much like oxygen, however as your body neglects to get the oxygen it needs, you might begin to feel cerebral pains, discombobulation, disarray, pass out, or even pass on.
6.Is Carbon Dioxide Poisonous?
Indeed, carbon dioxide is poisonous.
To be viewed as a poisonous to people, it is a substance that causes genuine actual damage or demise assuming it is ingested, inhaled, or in any case taken into the body.
CO2 can cause you hurt if you breath it, particularly in the event that you breath a great deal of it and for a drawn out timeframe.
-
Presently, consider this. The actual gas doesn’t cause harm. It is only the way that it replaces the oxygen the body needs.
-
At the point when you take in CO2 gas, it can cause genuine actual damage or demise, so it fits in inside the definition we use for harmfulness.
-
However, it doesn’t act the same way as other poisonous substances (like).
7.Is carbon dioxide is heterogeneous or homogeneous?
Since carbon dioxide is broken down in water, we can construe from the conduct of salt gems disintegrated in water that carbon dioxide disintegrated in water is (additionally) a homogeneous combination.
8. Why is carbon dioxide homogeneous?
Sometimes gases can be dissolved in liquids. For example, carbonated drinks have carbon dioxide dissolved in water, making a homogeneous mixture. Water is a chemical substance that contains only molecules made of two hydrogen atoms and one oxygen atom, so it’s a compound. It’s a heterogeneous mixture.
9. Is carbon dioxide considered a pure substance?
It is not a pure substance. Carbon dioxide is a compound while oxygen and zinc are elements which are a type of pure substance.
10.Why is carbon dioxide a compound?
Carbon dioxide is a chemical compound Electron dot structures of co2 of one carbon and two oxygen atoms. It is often referred to by its formula CO2. It is present in the Earth’s atmosphere at a low concentration and acts as a greenhouse gas.
Conclusion
If anyone confused that Is carbon dioxide a pure substance? And want to know correct information. Then, I suggest that you must read this article carefully. Here, I described all details about Is carbon dioxide a pure substance.
Related Articles
You May Also Like;