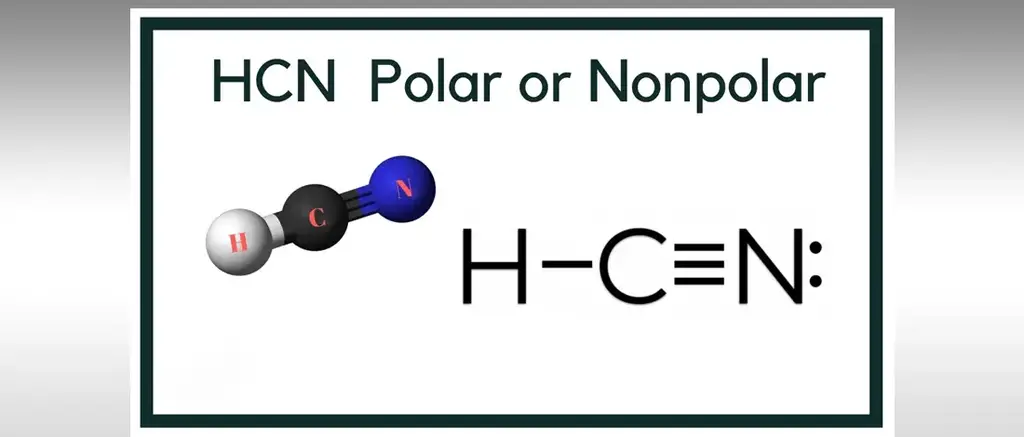
Is HCN Polar or Nonpola?HCN is a polar particle on the grounds that there is an enormous electronegative distinction between the N and H across the direct atom. It comprises of two polar bonds whose polarities line up in the equivalent direction. HCN is also known as hydrogen cyanide,
Is HCN Polar or Nonpolar?
Hydrogen cyanide is a substance compound with its engineered condition HCN. it is generally called prussic destructive. It is a destructive gas followed through on a cutting edge scale. We will analyze its properties and huge quantities of you may in like manner have questions concerning whether or not HCN is polar.
Thusly, I will make you grasp whether or not HCN is polar and the reason for that.
Things being what they are, is HCN polar or Nonpolar?
This substance compound has its nuclear mass of 27.0253 g/mol. It will in still up in the air as under:
Mol mass of HCN = 1 (Mol mass of H) + 1 (Mol mass of C) + 1 (Mol mass of N) = 1 + 12 + 14 =27 g/mol.
![]() The substance design of this engineered compound is covered by 1 carbon, 1 hydrogen, and 1 nitrogen particle.
The substance design of this engineered compound is covered by 1 carbon, 1 hydrogen, and 1 nitrogen particle.
![]() Carbon is the focal molecule encircled by nitrogen and hydrogen particles on the two sides with the end goal that it frames a direct shape structure.
Carbon is the focal molecule encircled by nitrogen and hydrogen particles on the two sides with the end goal that it frames a direct shape structure.
![]() The hydrogen has a valency of 1 (needs 1 electron more to get steady) and carbon has 4 valence electrons a requires 4 additional to finish its octet and nitrogen has 5 valence electrons and necessities 3 electrons more to finish its octet.
The hydrogen has a valency of 1 (needs 1 electron more to get steady) and carbon has 4 valence electrons a requires 4 additional to finish its octet and nitrogen has 5 valence electrons and necessities 3 electrons more to finish its octet.
![]() As needs be, carbon and hydrogen share electrons of one another and structures a covalent bond(C-H) though carbon and nitrogen structure a triple bond (C≡N) to impart their three electrons to one another.
As needs be, carbon and hydrogen share electrons of one another and structures a covalent bond(C-H) though carbon and nitrogen structure a triple bond (C≡N) to impart their three electrons to one another.
Subsequently, the particle H-C≡N becomes settled.
![]() Assuming we check the electronegativity of its iotas, the electronegativity of carbon is 2.55, nitrogen is 3.04, and that hydrogen is 2.2.
Assuming we check the electronegativity of its iotas, the electronegativity of carbon is 2.55, nitrogen is 3.04, and that hydrogen is 2.2.
![]() Furthermore nitrogen and carbon iotas are at outrageous positions and have a calculable contrast in their electronegativity.
Furthermore nitrogen and carbon iotas are at outrageous positions and have a calculable contrast in their electronegativity.
![]() Thus, the nitrogen acquires a halfway regrettable charge while the hydrogen acquires a fractional positive charge. This makes positive and negative posts across the particle making it a polar atom.
Thus, the nitrogen acquires a halfway regrettable charge while the hydrogen acquires a fractional positive charge. This makes positive and negative posts across the particle making it a polar atom.
Polar versus Nonpolar Atoms
![]() The polar atoms are those particles that have positive and negative shafts produced across them.
The polar atoms are those particles that have positive and negative shafts produced across them.
![]() The nonpolar particles have no shafts created across it and have equivalent charge scattered among its iotas.
The nonpolar particles have no shafts created across it and have equivalent charge scattered among its iotas.
![]() The polar atoms have their dipole second worth equivalents non-zero. The appropriation of charge among its particles is non-uniform.
The polar atoms have their dipole second worth equivalents non-zero. The appropriation of charge among its particles is non-uniform.
![]() The covalent bond framed by two particles is supposed to be polar on the off chance that their electronegativity contrasts from one another.
The covalent bond framed by two particles is supposed to be polar on the off chance that their electronegativity contrasts from one another.
![]() This is on the grounds that a more electronegative iota pulls the reinforced electron pair towards its side and gains halfway regrettable charge and the other molecule acquires incomplete positive charge.
This is on the grounds that a more electronegative iota pulls the reinforced electron pair towards its side and gains halfway regrettable charge and the other molecule acquires incomplete positive charge.
![]() Instances of polar atoms are HCl, OF2, and so on You can really take a look at the justification for the extremity of HCl.
Instances of polar atoms are HCl, OF2, and so on You can really take a look at the justification for the extremity of HCl.
![]() The dipole snapshot of nonpolar atoms is dependably zero. Since in these atoms, the dispersion of charge is dependably uniform across the whole particle.
The dipole snapshot of nonpolar atoms is dependably zero. Since in these atoms, the dispersion of charge is dependably uniform across the whole particle.
![]() The covalent bond framed by two iotas is supposed to be nonpolar in the event that the electronegativity of the two particles is equivalent.
The covalent bond framed by two iotas is supposed to be nonpolar in the event that the electronegativity of the two particles is equivalent.
![]() Instances of nonpolar particles are Hexane, BF3, and so on You can look at the justification for the non-extremity of BF3.
Instances of nonpolar particles are Hexane, BF3, and so on You can look at the justification for the non-extremity of BF3.
For what reason is HCN a Polar Particle?
![]() The particle of HCN is polar as it contains the iotas (hydrogen, nitrogen, and carbon) that vary in their electronegativity.
The particle of HCN is polar as it contains the iotas (hydrogen, nitrogen, and carbon) that vary in their electronegativity.
![]() The electronegativity of an iota is a significant boundary to check assuming it is polar or not.
The electronegativity of an iota is a significant boundary to check assuming it is polar or not.
![]() In basic words, the electronegativity of a molecule is its ability to pull the electron towards its side.
In basic words, the electronegativity of a molecule is its ability to pull the electron towards its side.
![]() Thus, a more prominent electronegative particle pulls fortified electron pair to its side with more impact and brings about charge awkwardness.
Thus, a more prominent electronegative particle pulls fortified electron pair to its side with more impact and brings about charge awkwardness.
![]() Accordingly, a more prominent electronegative particle acquires incomplete negative charge because of more charge force on it.
Accordingly, a more prominent electronegative particle acquires incomplete negative charge because of more charge force on it.
![]() The distinction between the electronegativity of nitrogen and hydrogen is (3.04 - 2.2= 0.84) which is adequate to bring extremity up in the HCN atom.
The distinction between the electronegativity of nitrogen and hydrogen is (3.04 - 2.2= 0.84) which is adequate to bring extremity up in the HCN atom.
![]() The state of this particle is straight and has a net dipole towards nitrogen.
The state of this particle is straight and has a net dipole towards nitrogen.
![]() Aside from the electronegativity factor, the nitrogen is associated with carbon with a triple bond that additionally expands the force of charge on the nitrogen particle and makes the atom polar.
Aside from the electronegativity factor, the nitrogen is associated with carbon with a triple bond that additionally expands the force of charge on the nitrogen particle and makes the atom polar.
Central issues to decide the extremity of an atom
![]() There exist a few boundaries that ought to be remembered while really looking at the extremity of a particle. You should note down the beneath focuses and notice them
There exist a few boundaries that ought to be remembered while really looking at the extremity of a particle. You should note down the beneath focuses and notice them
Electronegativity: Assuming there is a covalent bond framed between two particles varying in their electronegativity, then, at that point, the higher electronegative iota pulls the electron somewhat more towards its side.
![]() Accordingly, the bond framed is polar. Assuming there is a contrast between the electronegativity of iotas engaged with a particle, the atom framed is polar in nature.
Accordingly, the bond framed is polar. Assuming there is a contrast between the electronegativity of iotas engaged with a particle, the atom framed is polar in nature.
![]() The distinction in electronegativity is straightforwardly corresponding to the extremity of the atom.
The distinction in electronegativity is straightforwardly corresponding to the extremity of the atom.
![]() On account of H-C≡N, Nitrogen is more electronegative than hydrogen and carbon turns into the negative shaft.
On account of H-C≡N, Nitrogen is more electronegative than hydrogen and carbon turns into the negative shaft.
Mathematical shape: assuming the state of a particle is twisted or hilter kilter, the charge across the atom is unevenly circulated and brings about a polar atom.
![]() While the evenly molded particle is nonpolar provided that the electronegativity of molecules is equivalent. Assuming it bungles, the particle can be polar.
While the evenly molded particle is nonpolar provided that the electronegativity of molecules is equivalent. Assuming it bungles, the particle can be polar.
![]() Like on account of HCN, albeit the state of the particle is symmetric (direct), the particle is polar because of the distinction in electronegativity of its molecules.
Like on account of HCN, albeit the state of the particle is symmetric (direct), the particle is polar because of the distinction in electronegativity of its molecules.
The following shows of the mathematical construction of the HCN atom.
Dipole Second:
![]() The dipole of a particle is the proportion of its extremity. The more noteworthy the extremity of an atom more is its extremity.
The dipole of a particle is the proportion of its extremity. The more noteworthy the extremity of an atom more is its extremity.
![]() It is the result of charge on iotas and the distance between the focuses of positive and negative charge.
It is the result of charge on iotas and the distance between the focuses of positive and negative charge.
D = Q*R
![]() It is meant by D. The dipole of the HCN particle is 2.98 Debye. Debye is its SI.
It is meant by D. The dipole of the HCN particle is 2.98 Debye. Debye is its SI.
Properties of HCN
-
It exists as a lackluster fluid at room temperature with a sleek scent.
-
It is harmful and combustible in nature created over a wide scope of enterprises.
-
It is acidic in nature and has a corrosiveness of 9.21 PKA.
-
The dissolving point of this substance is −13.29 °C or 8.08 °F, and its edge of boiling over is 26 °C or 79 °F.
-
At a temperature of 25 °C, its fume pressure is 100 kPa.
-
The extremity of HCN is 2.98 D.
-
The atomic state of HCN is straight.
Employments of HCN
![]() HCN is utilized in the arrangement of acrylonitrile which is additionally utilized in the assembling of engineered rubbers, acrylic strands.
HCN is utilized in the arrangement of acrylonitrile which is additionally utilized in the assembling of engineered rubbers, acrylic strands.
![]() It is likewise utilized in the creation of plastics.
It is likewise utilized in the creation of plastics.
![]() HCN and compounds shaped with this are helpful for some synthetic responses. For instance, itis utilized in the solidifying of steel and iron.
HCN and compounds shaped with this are helpful for some synthetic responses. For instance, itis utilized in the solidifying of steel and iron.
![]() This compound is additionally utilized during the time spent electroplating.
This compound is additionally utilized during the time spent electroplating.
HCN Lewis Design, Sub-atomic Calculation, Shape, and Extremity
![]() Hydrogen Cyanide is a boring, combustible, and toxic synthetic fluid. Addressed by the substance equation, HCN is one of those particles that has an intriguing Lewis structure. This fluid is utilized in electroplating, mining, and as an antecedent for a considerable length of time.
Hydrogen Cyanide is a boring, combustible, and toxic synthetic fluid. Addressed by the substance equation, HCN is one of those particles that has an intriguing Lewis structure. This fluid is utilized in electroplating, mining, and as an antecedent for a considerable length of time.
![]() Also to additionally comprehend Hydrogen Cyanide’s actual properties, it is fundamental to realize its Lewis structure and atomic calculation. Continue to peruse this post to discover its shape, extremity, and that’s only the tip of the iceberg. To start with, let us check out its Lewis speck structure and the valence electrons that take an interest in framing bonds.
Also to additionally comprehend Hydrogen Cyanide’s actual properties, it is fundamental to realize its Lewis structure and atomic calculation. Continue to peruse this post to discover its shape, extremity, and that’s only the tip of the iceberg. To start with, let us check out its Lewis speck structure and the valence electrons that take an interest in framing bonds.
HCN Valence Electrons
![]() To draw the Lewis spot construction of any particle, it is crucial for know the absolute number of valence electrons in the design. To know the valence electrons of HCN, let us go through the valence electrons of individual particles in Hydrogen Cyanide.
To draw the Lewis spot construction of any particle, it is crucial for know the absolute number of valence electrons in the design. To know the valence electrons of HCN, let us go through the valence electrons of individual particles in Hydrogen Cyanide.
![]() This particle is comprised of three unique molecules: Hydrogen, Carbon, and Nitrogen.
This particle is comprised of three unique molecules: Hydrogen, Carbon, and Nitrogen.
A.Lewis Structures and the Shapes of Molecules
Covalent Bonds and Lewis Structures
At the point when components join, there are two sorts of bonds that might shape between them:
-
Ionic bonds result from an exchange of electrons from one animal categories (generally a metal) to another (normally a nonmetal or polyatomic particle).
-
Covalent bonds result from a sharing of electrons by at least two particles (typically nonmetals).
-
Lewis hypothesis (Gilbert Newton Lewis, 1875-1946) centers around the valence electrons, since the peripheral electrons are the ones that are most elevated in energy and farthest from the core, and are along these lines the ones that are generally presented to different molecules when bonds structure.
-
Lewis spot graphs for components are a convenient method of imagining valence electrons, and particularly, what electrons are accessible to be partaken in covalent bonds. The valence electrons are composed as dabs
-
Encompassing the image for the component: one dab is place on each side first, and when every one of the four positions are filled, the excess specks are combined with one of the principal set of spots, with a limit of two dabs put on each side.
-
Unpaired electrons address spots where electrons can be acquired in ionic mixtures, or electrons that can be shared to shape atomic mixtures. (The valence electrons of helium are better addressed by two matched specks, since in the respectable gases as a whole, the valence electrons are in filled shells, and are inaccessible for holding.)
-
Covalent bonds by and large structure when a nonmetal consolidates with another nonmetal. The two components in the bond are drawn to the unpaired valence electrons so unequivocally that neither can remove the electron from the other (in contrast to the case with ionic bonds), so the unpaired valence electrons are shared by the two molecules, shaping a covalent bond:
-
The common electrons behave like they have a place with the two iotas in the bond, and they tie the two particles together into an atom. The common electrons are typically addressed as a line (— ) between the fortified particles. (In Lewis structures, a line addresses two electrons.)
-
Particles will generally shape covalent bonds so as to fulfill the octet rule, with each iota encompassed by eight electrons. (Hydrogen is an exemption, since it is in column 1 of the occasional table, and just has the 1s orbital accessible in the ground state, which can just hold two electrons.)
-
The common sets of electrons are holding sets (addressed by lines in the drawings above). The unshared sets of electrons are solitary sets or nonbonding sets.
-
Each of the bonds shown up to this point have been single bonds, in which one sets of electrons is being shared. It is additionally conceivable to have twofold bonds, in which two sets of electrons are shared, and triple bonds, in which three sets of electrons are shared:
-
Different bonds are more limited and more grounded than their relating single bond partners.
B. Composing Lewis Designs for Particles
Rules for Composing Lewis Constructions
-
Count the all out number of valence electrons in the atom or polyatomic particle. (For instance, H2O has 2x1 + 6 = 8 valence electrons, CCl4 has 4 + 4x7 = 32 valence electrons.)
-
For anions, add one valence electron for every unit of negative charge; for cations, deduct one electron for every unit of positive charge. (For instance, NO3-has 5 + 3x6 + 1 = 24 valence electrons; NH4+ has 5 + 4+1 – 1 = 8 valence electrons.)
-
Place the iotas comparative with one another. For particles of the equation AXn, place the iota with the lower bunch number in the middle.
-
In the event that An and X are in a similar gathering, place the particle with the higher period number in the middle. (This places the most un-electronegative molecule in the middle.) H is NEVER UNDER ANY Conditions a focal iota.
-
Draw a solitary bond from every terminal iota to the focal particle. Each bond utilizes two valence electrons.
-
Circulate the excess valence electrons two by two so every particle acquires eight electrons (or 2 for H). Place the solitary sets on the terminal iotas first , and spot any excess valence electrons on the focal molecule.
-
The quantity of electrons in the last construction should approach the quantity of valence electrons from Stage 1.
-
Assuming a molecule actually doesn’t have an octet, move a solitary pair from a terminal iota in the middle of the terminal particle and the focal particle to make a twofold or triple bond. Utilize the conventional charge as a rule for setting various bonds:
-
Formal charge = valence – (½ holding e-) – (solitary pair e-)
-
The proper charge is the charge a molecule would have assuming the holding electrons were shared similarly.
-
The amount of the conventional charges should approach the charge on the species.
-
More modest conventional charges are better (more steady) than bigger ones.
-
The quantity of particles having formal charges ought to be limited.
-
Like charges on neighboring iotas are not alluring.
-
A more regrettable proper charge ought to live on a more electronegative molecule.
Examples
1. CH4 (methane)
8 valence electrons (4 + 4x1)
![]() Place the C in the middle, and associate the four H’s to it:
Place the C in the middle, and associate the four H’s to it:
![]() This uses up all of the valence electrons. The octet rule is fulfilled all over the place, and each of the molecules have formal charges of nothing.
This uses up all of the valence electrons. The octet rule is fulfilled all over the place, and each of the molecules have formal charges of nothing.
2. NH3 (ammonia)
8 valence electrons (5 + 3x1)
![]() Place the N in the middle, and interface the three H’s to it:
Place the N in the middle, and interface the three H’s to it:
![]() This uses up six of the eight valence electrons. The last two electrons can’t go on the H’s (that would disregard the octet rule for H), so they should go on the N:
This uses up six of the eight valence electrons. The last two electrons can’t go on the H’s (that would disregard the octet rule for H), so they should go on the N:
![]() All of the valence electrons have now been spent, the octet rule is fulfilled all over the place, and each of the particles have formal charges of nothing.
All of the valence electrons have now been spent, the octet rule is fulfilled all over the place, and each of the particles have formal charges of nothing.
3. H2O (water)
8 valence electrons (2x1 + 6)
![]() Place the O in the middle, and interface the two H’s to it:
Place the O in the middle, and interface the two H’s to it:
![]() This uses up four of the valence electrons. The leftover four valence electrons can’t go on the H’s, so they should go on the O, in two sets:
This uses up four of the valence electrons. The leftover four valence electrons can’t go on the H’s, so they should go on the O, in two sets:
![]() All of the valence electrons have now been spent, the octet rule is fulfilled all over the place, and each of the iotas have formal charges of nothing.
All of the valence electrons have now been spent, the octet rule is fulfilled all over the place, and each of the iotas have formal charges of nothing.
4. H3O+ (hydronium ion)
8 valence electrons (3x1 + 6 – 1)
![]() Place the O in the middle, and interface the three H’s to it:
Place the O in the middle, and interface the three H’s to it:
This uses up six of the valence electrons. The leftover two valence electrons should go on the oxygen:
![]() All of the valence electrons have been spent, and the octet rule is fulfilled all over the place. The proper charge on the oxygen iota is 1+ (8 – ½·6 – 2):
All of the valence electrons have been spent, and the octet rule is fulfilled all over the place. The proper charge on the oxygen iota is 1+ (8 – ½·6 – 2):
5. HCN (hydrogen cyanide)
10 valence electrons (1 + 4 + 5)
![]() Place the C in the middle, and interface the H and N to it:
Place the C in the middle, and interface the H and N to it:
This uses up four of the valence electrons. The leftover six valence electrons begin on the N:
![]() In the design as displayed, the octet rule isn’t fulfilled on the C, and there is a 2+ conventional charge on the C (4 – ½·4 – 0) and a 2-formal charge on the N (5 – ½·2 – 6):
In the design as displayed, the octet rule isn’t fulfilled on the C, and there is a 2+ conventional charge on the C (4 – ½·4 – 0) and a 2-formal charge on the N (5 – ½·2 – 6):
The octet rule can be fulfilled assuming that we move two sets of electrons from the N in the middle of the C and the N, making a triple bond:
The octet rule is currently fulfilled, and the conventional charges are zero.
6. CO2 (carbon dioxide)
16 valence electrons (4 + 2x6)
![]() Place the C in the middle, associate the two O’s to it, and spot the excess valence electrons on the O’s:
Place the C in the middle, associate the two O’s to it, and spot the excess valence electrons on the O’s:
![]() This uses up the sixteen valence electrons The octet rule isn’t fulfilled on the C, and there are heaps of formal charges in the construction:
This uses up the sixteen valence electrons The octet rule isn’t fulfilled on the C, and there are heaps of formal charges in the construction:
![]() The octet rule can be fulfilled, and the conventional charges lessened assuming that we move a couple of electrons from every oxygen molecule in the middle of the carbon and oxygen iotas:
The octet rule can be fulfilled, and the conventional charges lessened assuming that we move a couple of electrons from every oxygen molecule in the middle of the carbon and oxygen iotas:
![]() The octet rule is fulfilled all over the place, and every one of the iotas have formal charges of nothing.
The octet rule is fulfilled all over the place, and every one of the iotas have formal charges of nothing.
7. CCl4 (carbon tetrachloride)
32 valence electrons (4 + 4x7)
![]() Place the C in the middle, and interface the four Cl’s to it:
Place the C in the middle, and interface the four Cl’s to it:
This uses up eight valence electrons The leftover 24 valence electrons are put two by two on the Cl’s:
![]() Presently, all of the valence electrons have been spent, the octet rule is fulfilled all over the place, and every one of the iotas have formal charges of nothing.
Presently, all of the valence electrons have been spent, the octet rule is fulfilled all over the place, and every one of the iotas have formal charges of nothing.
8. COCl2 (phosgene or carbonyl chloride)
24 valence electrons (4 + 6 + 2x7)
![]() Place the C in the middle, and associate the O and the two Cl’s to it. (The overall situation of the O and the Cl’s doesn’t matter, since we are not yet drawing a three-layered design.)
Place the C in the middle, and associate the O and the two Cl’s to it. (The overall situation of the O and the Cl’s doesn’t matter, since we are not yet drawing a three-layered design.)
![]() Place the excess valence electrons on the O and Cl molecules:
Place the excess valence electrons on the O and Cl molecules:
![]() The octet rule isn’t fulfilled on the C; to get eight electrons around the C, we should move a couple of electrons either from the O or one of the Cl’s to make a twofold bond.
The octet rule isn’t fulfilled on the C; to get eight electrons around the C, we should move a couple of electrons either from the O or one of the Cl’s to make a twofold bond.
![]() Making a carbon-chlorine twofold bond would fulfill the octet rule, however there would in any case be formal charges, and there would be a positive proper charge on the firmly electronegative Cl molecule (structure 2).
Making a carbon-chlorine twofold bond would fulfill the octet rule, however there would in any case be formal charges, and there would be a positive proper charge on the firmly electronegative Cl molecule (structure 2).
![]() Making a carbon-oxygen twofold bond would likewise fulfill the octet rule, however every one of the proper charges would be zero, and that would be the better Lewis (structure 3):
Making a carbon-oxygen twofold bond would likewise fulfill the octet rule, however every one of the proper charges would be zero, and that would be the better Lewis (structure 3):
C. Resonance Structures — When One Lewis Structure Isn’t Enough
Examples (continued from section)
9. O3 (ozone)
18 valence electrons (3x6)
Place one O in the middle, and interface the other two O’s to it.
Drawing a solitary bond from the terminal O’s to the one in the middle uses four electrons; 12 of the leftover electrons go on the terminal O’s, leaving one solitary pair on the focal O:
We can fulfill the octet rule on the focal O by making a twofold bond either between the left O and the focal one (2), or the right O and the middle one (3):
The inquiry is, which one is the “right” Lewis structure?
In this model, we can draw two Lewis structures that are enthusiastically comparable to one another — that is, they have similar sorts of bonds, and similar kinds of formal charges on the designs as a whole. The two designs (2 and 3) should be utilized to address the particle’s construction.
The real atom is a normal of constructions 2 and 3, which are called reverberation structures. (Structure 1 is additionally a reverberation design of 2 and 3, however since it has more proper charges, and doesn’t fulfill the octet rule, it is a higher-energy reverberation structure, and doesn’t contribute as a lot to our general image of the particle.)
Structures 2 and 3 in the model above are to some degree “anecdotal” structures, in that they infer that there are “genuine” twofold bonds and single bonds in the design for ozone.
Actually, in any case, ozone has two oxygen-oxygen bonds which are equivalent long, and are somewhere between the lengths of run of the mill oxygen-oxygen single bonds and twofold bonds successfully, there are two “one-and-a-half” bonds in ozone.
The genuine atom doesn’t substitute to and fro between these two constructions; it is a half and half of these two structures.
This is similar to portraying a genuine individual as having the qualities of at least two anecdotal characters the anecdotal characters don’t exist, yet the genuine individual does. Another similarity is to think about a donkey: a donkey is a cross or crossover between a pony and a jackas, yet it doesn’t switch back and forth between being a pony.
The ozone atom, then, at that point, is all the more accurately displayed with both Lewis structures, with the two-headed reverberation bolt () between them:
In these reverberation structures, one of the electron sets (and consequently the negative charge) is “spread out” or delocalized over the entire particle. Conversely, the solitary sets on the oxygen in water are limited i.e., they’re caught in one spot.
Reverberation delocalization settles an atom by fanning out charges, and frequently happens when solitary sets (or positive charges) are situated close to twofold bonds. Reverberation assumes an enormous part in our comprehension of design and reactivity in natural science.
A more precise image of holding in atoms like this is found in Sub-atomic Orbital hypothesis, however this hypothesis is further developed, and numerically more intricate theme, and won’t be managed here.
When in doubt, when it’s feasible to make a twofold security in more than one area, and the subsequent designs are vigorously identical to one another, each different construction should be shown, isolated from one another by reverberation bolts.
Examples
10. CO32- (carbonate ion)
24 valence electrons (4 + 3x6 + 2)
![]() Place the C in the middle, with three solitary sets on every one of the O’s:
Place the C in the middle, with three solitary sets on every one of the O’s:
![]() We can fulfill the octet rule and make the proper charges more modest by making a carbon-oxygen twofold bond.
We can fulfill the octet rule and make the proper charges more modest by making a carbon-oxygen twofold bond.
![]() Since there are three vigorously identical methods of making a C=O, we draw every one of the three potential constructions, with a reverberation bolt between them:
Since there are three vigorously identical methods of making a C=O, we draw every one of the three potential constructions, with a reverberation bolt between them:
![]() By and by, structure 1 is a reverberation construction of 2, 3, and 4, however it is a higher energy structure, and doesn’t contribute as a lot to our image of the particle.
By and by, structure 1 is a reverberation construction of 2, 3, and 4, however it is a higher energy structure, and doesn’t contribute as a lot to our image of the particle.
![]() Since the twofold bond is fanned out north of three positions, the carbon-oxygen bonds in carbonate are “one-and-a-third” bonds.
Since the twofold bond is fanned out north of three positions, the carbon-oxygen bonds in carbonate are “one-and-a-third” bonds.
D. “Violations” of the Octet Rule
![]() Various species seem to disregard the octet rule by having less than eight electrons around the focal molecule, or by having in excess of eight electrons around the focal particle. Indeed, the proper charge is a decent rule to use to choose whether a “infringement” of the octet rule is OK.
Various species seem to disregard the octet rule by having less than eight electrons around the focal molecule, or by having in excess of eight electrons around the focal particle. Indeed, the proper charge is a decent rule to use to choose whether a “infringement” of the octet rule is OK.
![]() Electron lacking species, like beryllium (Be), boron (B), and aluminum (Al) can have less than eight electrons around the focal molecules, yet have no proper charge on that particle. Particles with electron insufficient focal molecules will quite often be genuinely responsive (numerous electron-inadequate species go about as Lewis acids).
Electron lacking species, like beryllium (Be), boron (B), and aluminum (Al) can have less than eight electrons around the focal molecules, yet have no proper charge on that particle. Particles with electron insufficient focal molecules will quite often be genuinely responsive (numerous electron-inadequate species go about as Lewis acids).
![]() Free revolutionaries contain an odd number of valence electrons. Therefore, one iota in the Lewis design will have an odd number of electrons, and won’t have a total octet in the valence shell.
Free revolutionaries contain an odd number of valence electrons. Therefore, one iota in the Lewis design will have an odd number of electrons, and won’t have a total octet in the valence shell.
These species are incredibly responsive. When drawing these mixtures, streamline the arrangement of bonds and the odd electron to limit formal charges; there are regularly a few potential reverberation structures than can be drawn.
![]() Extended valence shells are frequently found in nonmetals from period 3 or higher, like sulfur, phosphorus, and chlorine. These species can oblige in excess of 8 electrons by pushing “extra” electrons into void d orbitals.
Extended valence shells are frequently found in nonmetals from period 3 or higher, like sulfur, phosphorus, and chlorine. These species can oblige in excess of 8 electrons by pushing “extra” electrons into void d orbitals.
![]() For instance, sulfur’s valence shell contains 3s, 3p, and 3d orbitals (since sulfur is in line 3 of the occasional table, the valence shell is n=3); in any case, since there are just 16 electrons on a nonpartisan sulfur particle, the 3d orbitals are empty.
For instance, sulfur’s valence shell contains 3s, 3p, and 3d orbitals (since sulfur is in line 3 of the occasional table, the valence shell is n=3); in any case, since there are just 16 electrons on a nonpartisan sulfur particle, the 3d orbitals are empty.
![]() At the point when sulfur frames a compound with another component, the unfilled 3d orbitals can oblige extra electrons. Note that period 2 components Can’t have in excess of eight electrons, since the n=2 shell has no d orbitals to put “extra” electrons in.
At the point when sulfur frames a compound with another component, the unfilled 3d orbitals can oblige extra electrons. Note that period 2 components Can’t have in excess of eight electrons, since the n=2 shell has no d orbitals to put “extra” electrons in.
Examples
14. BF3 (boron trifluoride)
24 valence electrons (3 + 3x7)
![]() The octet rule is not satisfied on the B, but the formal charges are all zero.
The octet rule is not satisfied on the B, but the formal charges are all zero.
![]() In fact, trying to make a boron-fluorine double bond would put a positive formal charge on fluorine; since fluorine is highly electronegative, this is extremely unfavorable.
In fact, trying to make a boron-fluorine double bond would put a positive formal charge on fluorine; since fluorine is highly electronegative, this is extremely unfavorable.
15. NO (nitrogen monoxide, or nitric oxide)
11 valence electrons (5 + 6)
![]() In this structure, the formal charges are all zero, but the octet rule is not satisfied on the N. Since there are an odd number of electrons, there is no way to satisfy the octet rule.
In this structure, the formal charges are all zero, but the octet rule is not satisfied on the N. Since there are an odd number of electrons, there is no way to satisfy the octet rule.
![]() Nitric oxide is a free radical, and is an extremely reactive compound.
Nitric oxide is a free radical, and is an extremely reactive compound.
![]() In the body, nitric oxide is a vasodilator, and is involved in the mechanism of action of various neurotransmitters, as well as some heart and blood pressure medications such as nitroglycerin and amyl nitrite.
In the body, nitric oxide is a vasodilator, and is involved in the mechanism of action of various neurotransmitters, as well as some heart and blood pressure medications such as nitroglycerin and amyl nitrite.
16. PCl5 (phosphorus pentachloride)
40 valence electrons (5 + 5x7)
![]() The octet rule is violated on the central P, but phosphorus is in the p-block of row 3 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
The octet rule is violated on the central P, but phosphorus is in the p-block of row 3 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
Notice that the formal charge on the phosphorus atom is zero.
17. SF6 (sulfur hexafluoride)
48 valence electrons (6 + 6x7)
![]() The octet rule is violated on the central S, but sulfur is in the p-block of row 3 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
The octet rule is violated on the central S, but sulfur is in the p-block of row 3 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
![]() Notice that the formal charge on the sulfur atom is zero.
Notice that the formal charge on the sulfur atom is zero.
18. SF4 (sulfur tetrafluoride)\
48 valence electrons (6 + 6x7)
![]() The octet rule is violated on the central S, but sulfur is in the p-block of row 3 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
The octet rule is violated on the central S, but sulfur is in the p-block of row 3 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
![]() Notice that the formal charge on the sulfur atom is zero.
Notice that the formal charge on the sulfur atom is zero.
19. XeF4 (xenon tetrafluoride)
36 valence electrons (8 + 4x7)
![]() The octet rule is violated on the central Xe, but xenon is in the p-block of row 5 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
The octet rule is violated on the central Xe, but xenon is in the p-block of row 5 of the periodic table, and has empty d orbitals that can accommodate “extra” electrons.
![]() Notice that the formal charge on the xenon atom is zero.
Notice that the formal charge on the xenon atom is zero.
20. H2SO4 (sulfuric acid)
32 valence electrons (2x1 + 6 + 4x6)
![]() Structures 1 and 2 are resonance structures of each other, but structure 2 is the lower energy structure, even though it violates the octet rule.
Structures 1 and 2 are resonance structures of each other, but structure 2 is the lower energy structure, even though it violates the octet rule.
![]() Sulfur can accommodate more than eight electrons, and the formal charges in structure 2 are all zero.
Sulfur can accommodate more than eight electrons, and the formal charges in structure 2 are all zero.
Summary
To realize how the bonds are situated in space, you must have a solid handle of Lewis constructions and VSEPR hypothesis. Expecting you do, you can take a gander at the construction of every one and choose if it is polar or not - whether or not you know the singular molecule electronegativity. This is on the grounds that you realize that all connections between disparate components are polar, and in these specific models, it doesn’t make any difference which heading the dipole second vectors are bringing up (out or in).
E. The Shapes of Molecules: The VSEPR Model
![]() Drawing a Lewis structure is the initial moves towards anticipating the three-layered state of a particle. An atom’s shape unequivocally influences its actual properties and the manner in which it connects with different particles, and assumes a significant part in the way that organic atoms (proteins, catalysts, DNA, and so forth) associate.
Drawing a Lewis structure is the initial moves towards anticipating the three-layered state of a particle. An atom’s shape unequivocally influences its actual properties and the manner in which it connects with different particles, and assumes a significant part in the way that organic atoms (proteins, catalysts, DNA, and so forth) associate.
![]() The estimated state of an atom can be anticipated utilizing the Valence-Shell Electron-Pair Repugnance (VSEPR) model, which portrays electrons in bonds and solitary sets as “electron gatherings” that repulse each other and remain as far separated as could really be expected:
The estimated state of an atom can be anticipated utilizing the Valence-Shell Electron-Pair Repugnance (VSEPR) model, which portrays electrons in bonds and solitary sets as “electron gatherings” that repulse each other and remain as far separated as could really be expected:
![]() Draw the Lewis structure for the particle of interest and count the quantity of electron bunches encompassing the focal iota. Every one of the accompanying comprises an electron bunch:
Draw the Lewis structure for the particle of interest and count the quantity of electron bunches encompassing the focal iota. Every one of the accompanying comprises an electron bunch:
-
A solitary, twofold or triple bond (various bonds consider one electron bunch)
-
A solitary pair
-
An unpaired electron
![]() Anticipate the course of action of electron bunches around every particle by expecting that the gatherings are situated in space as distant from each other as could be expected.
Anticipate the course of action of electron bunches around every particle by expecting that the gatherings are situated in space as distant from each other as could be expected.
![]() The states of bigger particles having more than one focal are a composite of the states of the iotas inside the atom, every one of which can be anticipated utilizing the VSEPR model.
The states of bigger particles having more than one focal are a composite of the states of the iotas inside the atom, every one of which can be anticipated utilizing the VSEPR model.
![]() Solitary sets occupy more space than covalent bonds; this makes different molecules be crushed together somewhat, diminishing the bond points by a couple of degrees.
Solitary sets occupy more space than covalent bonds; this makes different molecules be crushed together somewhat, diminishing the bond points by a couple of degrees.
![]() The three-sided bipyramidal shape can be envisioned collectively of three securities in a three-sided planar course of action isolated by bond points of 120° (the tropical situations), with two additional bonds at a point of 90° to this plane (the pivotal positions):
The three-sided bipyramidal shape can be envisioned collectively of three securities in a three-sided planar course of action isolated by bond points of 120° (the tropical situations), with two additional bonds at a point of 90° to this plane (the pivotal positions):
![]() Solitary sets go in the tropical situations, since they occupy more space than covalent bonds. In the central position, solitary sets are ~120° from two different bonds, while in the pivotal positions they would be 90° away from three different bonds.
Solitary sets go in the tropical situations, since they occupy more space than covalent bonds. In the central position, solitary sets are ~120° from two different bonds, while in the pivotal positions they would be 90° away from three different bonds.
![]() With Lewis structures including reverberation, it is superfluous which construction is utilized to decide the shape, since they are altogether vigorously same.
With Lewis structures including reverberation, it is superfluous which construction is utilized to decide the shape, since they are altogether vigorously same.
F. Polar and Nonpolar Covalent Bonds
![]() Electronegativity is a proportion of the capacity of a particle in an atom to draw in shared electrons in a covalent bond. Electronegativity is an intermittent property, and increments from base to top inside a gathering and from left to right across a period:
Electronegativity is a proportion of the capacity of a particle in an atom to draw in shared electrons in a covalent bond. Electronegativity is an intermittent property, and increments from base to top inside a gathering and from left to right across a period:
![]() At the point when two iotas of a similar electronegativity share electrons, the electrons are shared similarly, and the bond is a nonpolar covalent bond — there is an even conveyance of electrons between the fortified particles. (As a similarity, you can consider it a round of back-and-forth between two similarly solid groups, in which the rope doesn’t move.)
At the point when two iotas of a similar electronegativity share electrons, the electrons are shared similarly, and the bond is a nonpolar covalent bond — there is an even conveyance of electrons between the fortified particles. (As a similarity, you can consider it a round of back-and-forth between two similarly solid groups, in which the rope doesn’t move.)
![]() For instance, when two chlorine particles are joined by a covalent bond, the electrons invest the same amount of energy near one chlorine iota as they do to the next; the subsequent atom is nonpolar (demonstrated by the even electron cloud displayed underneath):
For instance, when two chlorine particles are joined by a covalent bond, the electrons invest the same amount of energy near one chlorine iota as they do to the next; the subsequent atom is nonpolar (demonstrated by the even electron cloud displayed underneath):
![]() At the point when two reinforced particles have a distinction of more noteworthy than 2.0 electronegativity units (see Table 2), the bond is an ionic bond — one iotas removes the electrons from the other molecule, creating cations and anions.
At the point when two reinforced particles have a distinction of more noteworthy than 2.0 electronegativity units (see Table 2), the bond is an ionic bond — one iotas removes the electrons from the other molecule, creating cations and anions.
For instance Na has an electronegativity of 0.93, and Cl is 3.16, a distinction of 2.23 units. The Cl particle removes an electron from the Na, delivering a completely ionic bond:
![]() At the point when two fortified particles have a distinction of somewhere in the range of 0.4 and 2.0 electronegativity units (see Table 2), the electrons are shared inconsistent, and the bond is a polar covalent bond there is an unsymmetrical conveyance of electrons between the reinforced iotas.
At the point when two fortified particles have a distinction of somewhere in the range of 0.4 and 2.0 electronegativity units (see Table 2), the electrons are shared inconsistent, and the bond is a polar covalent bond there is an unsymmetrical conveyance of electrons between the reinforced iotas.
Since one molecule in the bond is “pulling” on the common electrons than the other, yet not hard enough to take the electrons totally away.
![]() The more electronegative particle in the bond has a halfway regrettable charge (δ-), in light of the fact that the electrons are pulled somewhat towards that iota, and the less electronegative molecule has a fractional positive charge (δ+), on the grounds that the electrons are incompletely (yet not totally) pulled away from that iota.
The more electronegative particle in the bond has a halfway regrettable charge (δ-), in light of the fact that the electrons are pulled somewhat towards that iota, and the less electronegative molecule has a fractional positive charge (δ+), on the grounds that the electrons are incompletely (yet not totally) pulled away from that iota.
![]() For instance, in the HCl particle, chlorine is more electronegative than hydrogen by 0.96 electronegativity units. The common electrons are pulled somewhat nearer to the chlorine iota, making the chlorine end of the particle marginally negative (showed in the figure beneath by the bigger electron cloud around the Cl molecule),
For instance, in the HCl particle, chlorine is more electronegative than hydrogen by 0.96 electronegativity units. The common electrons are pulled somewhat nearer to the chlorine iota, making the chlorine end of the particle marginally negative (showed in the figure beneath by the bigger electron cloud around the Cl molecule),
![]() While the hydrogen end of the particle is marginally certain (showed by the more modest electron cloud around the H iota), and the subsequent atom is polar:
While the hydrogen end of the particle is marginally certain (showed by the more modest electron cloud around the H iota), and the subsequent atom is polar:
![]() We say that the bond has a dipole the electron cloud is spellbound towards one finish of the atom. The level of extremity in a covalent bond relies upon the electronegativity distinction, EN, between the two reinforced iotas:
We say that the bond has a dipole the electron cloud is spellbound towards one finish of the atom. The level of extremity in a covalent bond relies upon the electronegativity distinction, EN, between the two reinforced iotas:
-
EN 0 - 0.4 = Nonpolar covalent bond
-
EN 0.4 - 2.0 = Polar covalent bond
-
EN > 2.0 = Ionic bond
G. Atomic Shape and Extremity
![]() In a diatomic atom (X2 or XY), there is just one bond, and the extremity of that bond decides the extremity of the particle: assuming the bond is polar, the atom is polar, and assuming the bond is nonpolar, the atom is nonpolar.
In a diatomic atom (X2 or XY), there is just one bond, and the extremity of that bond decides the extremity of the particle: assuming the bond is polar, the atom is polar, and assuming the bond is nonpolar, the atom is nonpolar.
![]() In atoms with more than one bond, both shape and bond extremity decide if the particle is polar.
In atoms with more than one bond, both shape and bond extremity decide if the particle is polar.
![]() A particle should contain polar bonds all together for the atom to be polar, yet assuming the polar bonds are adjusted precisely inverse to one another, or on the other hand on the off chance that they are adequately symmetric, the bond polarities counteract, making the particle nonpolar. (Extremity is a vector amount, so both the greatness and the course should be considered.)
A particle should contain polar bonds all together for the atom to be polar, yet assuming the polar bonds are adjusted precisely inverse to one another, or on the other hand on the off chance that they are adequately symmetric, the bond polarities counteract, making the particle nonpolar. (Extremity is a vector amount, so both the greatness and the course should be considered.)
![]() For instance, consider the Lewis dab structure for carbon dioxide. This is a straight atom, containing two polar carbon-oxygen twofold bonds. Be that as it may, since the polar bonds are poiExcluded values calculatornting precisely 180° away from one another.
For instance, consider the Lewis dab structure for carbon dioxide. This is a straight atom, containing two polar carbon-oxygen twofold bonds. Be that as it may, since the polar bonds are poiExcluded values calculatornting precisely 180° away from one another.
The bond polarities counterbalance, and the atom is nonpolar. (As a similarity, you can imagine this is resembling a round of back-and-forth between two groups that are pulling on a rope similarly hard.
![]() The water atom likewise contains polar bonds, however since it is a twisted particle, the bonds are at a point to one another of around 105°. They don’t counterbalance since they are not pointing precisely towards one another, and there is a general dipole going from the hydrogen end of the atom towards the oxygen end of the particle; water is in this manner a polar atom:
The water atom likewise contains polar bonds, however since it is a twisted particle, the bonds are at a point to one another of around 105°. They don’t counterbalance since they are not pointing precisely towards one another, and there is a general dipole going from the hydrogen end of the atom towards the oxygen end of the particle; water is in this manner a polar atom:
![]() Particles in which every one of the molecules encompassing the focal iota are the equivalent will generally be nonpolar assuming there are no solitary sets on the focal particle.
Particles in which every one of the molecules encompassing the focal iota are the equivalent will generally be nonpolar assuming there are no solitary sets on the focal particle.
Assuming that a portion of the iotas encompassing the focal particle are unique, notwithstanding, the atom might be polar. For instance, carbon tetrachloride, CCl4, is nonpolar, however chloroform, CHCl3, and methyl chloride, CH3Cl are polar:
![]() The extremity of a particle strongly affects its actual properties. Particles which are more polar have more grounded intermolecular powers among them, and have, by and large, higher limits (just as other distinctive actual properties).
The extremity of a particle strongly affects its actual properties. Particles which are more polar have more grounded intermolecular powers among them, and have, by and large, higher limits (just as other distinctive actual properties).
![]() The table beneath shows whether the models in the past areas are polar or nonpolar.
The table beneath shows whether the models in the past areas are polar or nonpolar.
For species which have a general charge, the expression “charged” is utilized all things being equal, since the expressions “polar” and “nonpolar” don’t actually apply to charged species; charged species are, by definition, basically polar. Solitary sets on some external particles have been excluded for clearness.
-
“Electron gatherings” incorporate securities, solitary sets, and odd (unpaired) electrons. A various bond (twofold bond or triple bond) considers one electron bunch.
-
A various bond (twofold bond or triple bond) includes as one bond in the VSEPR model.
-
A = focal molecule, X = encompassing particles, E = solitary sets
-
Particles with this shape are nonpolar when each of the molecules associated with the focal iota are something similar. Assuming the particles associated with the focal iota are not quite the same as one another, the atomic extremity should be considered dependent upon the situation.
-
Since electrons in solitary sets occupy more space than electrons in covalent bonds, when solitary sets are available the bond points are “crushed” somewhat contrasted with the fundamental construction without solitary sets.
Electronegativity and Bond Extremity
Bond Extremity
![]() The capacity of a particle in an atom to draw in shared electrons is called electronegativity. At the point when two iotas join, the distinction between their electronegativities means that the sort of bond that will shape.
The capacity of a particle in an atom to draw in shared electrons is called electronegativity. At the point when two iotas join, the distinction between their electronegativities means that the sort of bond that will shape.
![]() On the off chance that the distinction between the electronegativities of the two iotas is little, neither one of the particles can remove the common electrons totally from the other molecule and the bond will be covalent.
On the off chance that the distinction between the electronegativities of the two iotas is little, neither one of the particles can remove the common electrons totally from the other molecule and the bond will be covalent.
![]() Assuming the contrast between the electronegativities is enormous, the more electronegative molecule will remove the holding electrons totally from the other iota (electron move will happen) and the bond will be ionic.
Assuming the contrast between the electronegativities is enormous, the more electronegative molecule will remove the holding electrons totally from the other iota (electron move will happen) and the bond will be ionic.
![]() To this end metals (low electronegativities) reinforced with nonmetals (high electronegativities) regularly produce ionic mixtures.
To this end metals (low electronegativities) reinforced with nonmetals (high electronegativities) regularly produce ionic mixtures.
![]() A bond might be extremely polar that an electron really moves starting with one molecule then onto the next, shaping a genuine ionic bond. Researchers have conceived a scale called electronegativity, a scale for deciding how much iotas of any component draw in electrons.
A bond might be extremely polar that an electron really moves starting with one molecule then onto the next, shaping a genuine ionic bond. Researchers have conceived a scale called electronegativity, a scale for deciding how much iotas of any component draw in electrons.
![]() Electronegativity is a unitless number; the higher the number, the more a molecule draws in electrons. A typical scale for electronegativity is displayed in Figure 5.10.15.10.1.
Electronegativity is a unitless number; the higher the number, the more a molecule draws in electrons. A typical scale for electronegativity is displayed in Figure 5.10.15.10.1.
The extremity of a covalent bond can be decided by deciding the distinction of the electronegativities of the two molecules engaged with the covalent bond, as summed up in the accompanying table:
Electronegativity Difference Bond Type 0 nonpolar covalent 0–0.4 slightly polar covalent 0.5–2.1 definitely polar covalent >2.1 likely ionic
Nonpolar Covalent Bonds
![]() A bond in which the electronegativity contrast is under 1.9 is viewed as generally covalent in character. In any case, now, we really want to recognize two general sorts of covalent bonds. A nonpolar covalent bond is a covalent bond where the holding electrons are shared similarly between the two iotas. In a nonpolar covalent bond, the dissemination of electrical charge is adjusted between the two iotas.
A bond in which the electronegativity contrast is under 1.9 is viewed as generally covalent in character. In any case, now, we really want to recognize two general sorts of covalent bonds. A nonpolar covalent bond is a covalent bond where the holding electrons are shared similarly between the two iotas. In a nonpolar covalent bond, the dissemination of electrical charge is adjusted between the two iotas.
![]() The two chlorine particles share the pair of electrons in the single covalent bond similarly, and the electron thickness encompassing the Cl2Cl2 atom is balanced.
The two chlorine particles share the pair of electrons in the single covalent bond similarly, and the electron thickness encompassing the Cl2Cl2 atom is balanced.
![]() Additionally note that atoms in which the electronegativity distinction is tiny (<0.5) are likewise thought to be nonpolar covalent. A model would be a connection among chlorine and bromine (ΔΔEN =3.0−2.8=0.2=3.0−2.8=0.2).
Additionally note that atoms in which the electronegativity distinction is tiny (<0.5) are likewise thought to be nonpolar covalent. A model would be a connection among chlorine and bromine (ΔΔEN =3.0−2.8=0.2=3.0−2.8=0.2).
Polar Covalent Bonds
![]() A bond in which the electronegativity distinction between the iotas is somewhere in the range of 0.5 and 2.1 is known as a polar covalent bond.
A bond in which the electronegativity distinction between the iotas is somewhere in the range of 0.5 and 2.1 is known as a polar covalent bond.
![]() A polar covalent bond is a covalent bond where the iotas have an inconsistent fascination for electrons thus the sharing is inconsistent. In a polar covalent bond, now and again basically called a polar bond, the conveyance of electrons around the particle is at this point not even.
A polar covalent bond is a covalent bond where the iotas have an inconsistent fascination for electrons thus the sharing is inconsistent. In a polar covalent bond, now and again basically called a polar bond, the conveyance of electrons around the particle is at this point not even.
![]() A simple method for showing the lopsided electron circulation in a polar covalent bond is to utilize the Greek letter delta (δ)(δ).
A simple method for showing the lopsided electron circulation in a polar covalent bond is to utilize the Greek letter delta (δ)(δ).
![]() The particle with the more prominent electronegativity secures a halfway regrettable charge, while the molecule with the lesser electronegativity obtains a fractional positive charge.
The particle with the more prominent electronegativity secures a halfway regrettable charge, while the molecule with the lesser electronegativity obtains a fractional positive charge.
![]() The delta image is utilized to demonstrate that the amount of charge is short of what one. A crossed bolt can likewise be utilized to demonstrate the course of more prominent electron thickness.
The delta image is utilized to demonstrate that the amount of charge is short of what one. A crossed bolt can likewise be utilized to demonstrate the course of more prominent electron thickness.
What is the extremity of each bond?
-
C–H
-
O–H
Arrangement
![]() Utilizing Figure 5.10.15.10.1, we can work out the distinction of the electronegativities of the molecules associated with the bond.
Utilizing Figure 5.10.15.10.1, we can work out the distinction of the electronegativities of the molecules associated with the bond.
![]() For the C–H bond, the distinction in the electronegativities is 2.5 − 2.1 = 0.4. Hence we foresee that this bond will be non polar covalent.
For the C–H bond, the distinction in the electronegativities is 2.5 − 2.1 = 0.4. Hence we foresee that this bond will be non polar covalent.
![]() For the O–H bond, the distinction in electronegativities is 3.5 − 2.1 = 1.4, so we anticipate that this bond will be most certainly polar covalent.
For the O–H bond, the distinction in electronegativities is 3.5 − 2.1 = 1.4, so we anticipate that this bond will be most certainly polar covalent.
Atomic Extremity
![]() To decide whether an atom is polar or nonpolar, it is regularly valuable to see Lewis structures. Nonpolar mixtures will be symmetric, which means every one of the sides around the focal particle are indistinguishable - attached to similar component with no unshared sets of electrons.
To decide whether an atom is polar or nonpolar, it is regularly valuable to see Lewis structures. Nonpolar mixtures will be symmetric, which means every one of the sides around the focal particle are indistinguishable - attached to similar component with no unshared sets of electrons.
![]() Polar particles are lopsided, either containing solitary sets of electrons on a focal molecule or having iotas with various electronegativities fortified. This functions admirably - as long as you can picture the sub-atomic calculation. That is the critical step.
Polar particles are lopsided, either containing solitary sets of electrons on a focal molecule or having iotas with various electronegativities fortified. This functions admirably - as long as you can picture the sub-atomic calculation. That is the critical step.
![]() To realize how the bonds are situated in space, you must have a solid handle of Lewis designs and VSEPR hypothesis. Accepting you do, you can take a gander at the construction of every one and choose if it is polar or not - whether or not you know the singular iota electronegativity.
To realize how the bonds are situated in space, you must have a solid handle of Lewis designs and VSEPR hypothesis. Accepting you do, you can take a gander at the construction of every one and choose if it is polar or not - whether or not you know the singular iota electronegativity.
![]() This is on the grounds that you realize that all connections between disparate components are polar, and in these specific models, it doesn’t make any difference which bearing the dipole second vectors are bringing up (out or in).
This is on the grounds that you realize that all connections between disparate components are polar, and in these specific models, it doesn’t make any difference which bearing the dipole second vectors are bringing up (out or in).
![]() A polar particle is an atom wherein one finish of the particle is somewhat certain, while the opposite end is marginally negative. A diatomic atom that comprises of a polar covalent bond, like HFHF, is a polar particle.
A polar particle is an atom wherein one finish of the particle is somewhat certain, while the opposite end is marginally negative. A diatomic atom that comprises of a polar covalent bond, like HFHF, is a polar particle.
![]() The two electrically charged locales on one or the flip side of the atom are called poles, like a magnet having a north and a south pole. An atom with two shafts is known as a dipole (see figure underneath). Hydrogen fluoride is a dipole.
The two electrically charged locales on one or the flip side of the atom are called poles, like a magnet having a north and a south pole. An atom with two shafts is known as a dipole (see figure underneath). Hydrogen fluoride is a dipole.
![]() For particles with multiple iotas, the sub-atomic calculation should likewise be considered while deciding whether the particle is polar or nonpolar. The figure underneath shows an examination between carbon dioxide and water.
For particles with multiple iotas, the sub-atomic calculation should likewise be considered while deciding whether the particle is polar or nonpolar. The figure underneath shows an examination between carbon dioxide and water.
Smith Machine Bar Weight
![]() Carbon dioxide (CO2)(CO2) is a direct particle. The oxygen iotas are more electronegative than the carbon particle, so there are two individual dipoles pointing outward from the CC molecule to each OO particle.
Carbon dioxide (CO2)(CO2) is a direct particle. The oxygen iotas are more electronegative than the carbon particle, so there are two individual dipoles pointing outward from the CC molecule to each OO particle.
![]() In any case, since the dipoles are of equivalent strength and are arranged along these lines, they counterbalance and the generally speaking sub-atomic extremity of CO2CO2 is zero.
In any case, since the dipoles are of equivalent strength and are arranged along these lines, they counterbalance and the generally speaking sub-atomic extremity of CO2CO2 is zero.
![]() Water is a bowed particle in light of the two solitary sets on the focal oxygen iota. The singular dipoles point from the HH molecules toward the OO iota. On account of the shape, the dipoles don’t counterbalance one another and the water atom is polar.
Water is a bowed particle in light of the two solitary sets on the focal oxygen iota. The singular dipoles point from the HH molecules toward the OO iota. On account of the shape, the dipoles don’t counterbalance one another and the water atom is polar.
![]() Some different particles are displayed in the figure underneath. Notice that a tetrahedral particle, for example, CH4CH4 is nonpolar. Nonetheless, assuming one of the fringe HH particles is supplanted with another iota that has an alternate electronegativity, the atom becomes polar.
Some different particles are displayed in the figure underneath. Notice that a tetrahedral particle, for example, CH4CH4 is nonpolar. Nonetheless, assuming one of the fringe HH particles is supplanted with another iota that has an alternate electronegativity, the atom becomes polar.
![]() A three-sided planar particle (BF3)(BF3) might be nonpolar assuming that each of the three fringe iotas are something very similar, yet a three-sided pyramidal atom (NH3)(NH3) is polar.
A three-sided planar particle (BF3)(BF3) might be nonpolar assuming that each of the three fringe iotas are something very similar, yet a three-sided pyramidal atom (NH3)(NH3) is polar.
To sum up, to be polar, an atom must:
-
Contain no less than one polar covalent bond.
-
Have an atomic construction with the end goal that the amount of the vectors of each bond dipole second doesn’t drop.
-
Steps to Recognize Polar Particles
-
Draw the Lewis structure
-
Sort out the math (utilizing VSEPR hypothesis)
-
Picture or draw the calculation
-
Track down the net dipole second (you don’t need to definitely do computations on the off chance that you can envision it)
-
On the off chance that the net dipole second is zero, it is non-polar. In any case, it is polar.
Properties of Polar Particles
-
Polar particles will more often than not adjust when set in an electric field with the positive finish of the atom situated toward the negative plate and the adverse end toward the positive plate .
-
We can utilize an electrically charged item to draw in polar atoms, yet nonpolar particles are not drawn in. Likewise, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances.
-
While atoms can be portrayed as “polar covalent” or “ionic”, it should be noticed that this is regularly a relative term, with one particle essentially being more polar or less polar than another. In any case, the accompanying properties are run of the mill of such atoms. Polar atoms tend to:
-
have higher liquefying focuses than nonpolar atoms
-
have higher limits than nonpolar particles
-
be more solvent in water (disintegrate better) than nonpolar particles
-
have lower fume pressures than nonpolar atoms
Model
Name every one of the accompanying as polar or nonpolar.
-
Water, H2O:
-
Methanol, CH3OH:
-
Hydrogen Cyanide, HCN:
-
Oxygen, O2:
-
Propane, C3H8:
Arrangement
-
Water is polar. Any particle with solitary sets of electrons around the focal molecule is polar.
-
Methanol is polar. This is anything but a symmetric atom. The −OH−OH side is not quite the same as the other 3 −H−H sides.
-
Hydrogen cyanide is polar. The atom isn’t symmetric. The nitrogen and hydrogen have various electronegativities, making a lopsided draw on the electrons.
-
Oxygen is nonpolar. The atom is symmetric. The two oxygen particles pull on the electrons by the very same sum.
-
Propane is nonpolar, on the grounds that it is symmetric, with HH iotas attached to each side around the focal molecules and no unshared sets of electrons.
Summary
To decide whether an atom is polar or nonpolar, it is habitually valuable to see Lewis structures. Nonpolar mixtures will be symmetric, which means every one of the sides around the focal molecule are indistinguishable - clung to similar component with no unshared sets of electrons. Polar particles are awry, either containing solitary sets of electrons on a focal molecule or having iotas with various electronegativities fortified. This functions admirably - as long as you can envision the sub-atomic calculation. That is the critical step.
Frequently Ask Questions
Here, some questions described related to this article:
1. What type of bond is HCN?
In HCN, Carbon is bonded to Nitrogen with a triple covalent bond consisting of one sigma bond and two pi bonds. The sigma bond is formed by overlapping hybridized orbitals, with the two remaining unhybridized orbitals overlapping to form the two pi bonds.
2. Is HCN soluble in water?
Hydrogen cyanide has a weak, harsh almond scent and a severe, consuming taste. It is solvent in water and is frequently utilized as a 96% fluid arrangement.
3. What is the electro cynicism contrast of HCN?
Contrasts in electronegativities are determined for each bond in a particle. For HCN you will ascertain the contrast among H and C which is 0.4 and the distinction among C and N which is 0.5.
4. Is HCN twisted or straight?
Hydrogen cyanide is a direct atom. A Lewis detailing counts 1 electron from the hydrogen, 4 electron from the carbon, and 5 electron from the nitrogen, so 5 electron sets to circulate.
5. Is HCN dissolvable in a nonpolar dissolvable?
HCN is solvent in water because of the accompanying reasons. It is polar in nature which implies it has some worth of dipole second.
6. Is HCN a solid base?
Frail acids, as solid acids, ionize to yield the H+ particle and a form base. Since HCl is a solid corrosive, its form base (Cl−) is very feeble. Solid and Powerless Acids and Corrosive Ionization Steady.
-
Corrosive
-
Form Base
-
HCN (hydrocyanic corrosive) (most vulnerable)
-
CN− (cyanide particle) (most grounded)
7. Is HCN a three-sided planar?
HCN just has two electron-thick regions around the focal particle; hence, it can’t be three-sided planar in shape.
8. What is the intermolecular force of ch3cooh?
In acidic corrosive (CH3COOH), hydrogen holding, dipole-dipole associations and scattering power are available though in carbon tetrachloride (CCl4) just scattering non-polar powers are available.
9. How many double bonds are in HCN?
Assuming we draw the Lewis structure for hydrogen cyanide, we will see that there are no twofold bonds present in HCN. HCN has an aggregate of ten (10) valence.
10. For what reason does HCN have no dipole second?
HCN is a straight particle; it has a super durable dipole second; it contains N, but the nitrogen isn’t straightforwardly clung to a hydrogen. Accordingly scattering powers and dipole-dipole powers act between sets of HCN atoms.
Conclusion
Polar atoms will quite often adjust. When put in an electric field with the positive finish of the particle arranged toward the negative plate and the adverse end toward the positive plate. We can utilize an electrically charged item to draw in polar atoms, however nonpolar particles are not drawn in. Additionally, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances. HCN, or hydrogen cyanide, is a polar particle on the grounds that there is an enormous electronegative distinction between the N and H across the direct atom. It comprises of two polar bonds whose polarities line up in the equivalent direction.