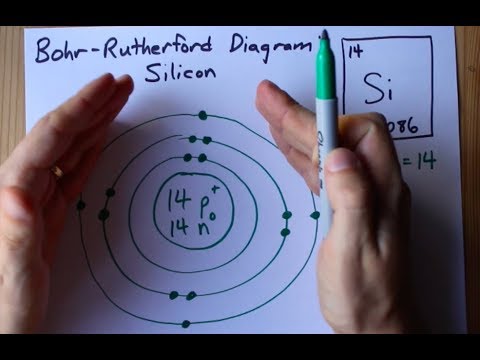
How Many Valence Electrons Are In Si? There are four valence electrons in a Si. Si have 14 atomic number which implies it has two electrons in its first shell, eight electrons in the subsequent shell, and four electrons in the third shell. The symbol of silicon is Si.
What Are Atomic Properties Of Silicon?
Following are basic atomic properties of silicon atom.
-
The electronic configuration of Si (Z = 14) = [Ne]3s2 3p2
-
Silicon is component 14 in the Periodic Table
-
It consist of two electrons in its first shell, then eight electrons in the second shell, and four electrons residing in the third shell.
Atomic Structure Of Silicon
Silicon atomic radius is 111 pm, while its covalent radius is 111 pm.
| 1. Atomic Radius | 111 pm (1.11 Å) |
|---|---|
| 2. Atomic Volume | 12.0538626609 cm3 |
| 3. Covalent Radius | 111 pm (1.11 Å) |
| 4. Van der Waals Radius | 210 pm |
| 5. Neutron Cross Section | 171 σa/barns |
Atomic Spectrum Of Silicon
Silicon Chemical Properties: Ionization Energies and electron affinity
| 1. The electron affinity of Silicon | 133.6 kJ/mol |
|---|---|
| 2. Electronegativity | 1.9 |
| 3. Electron Affinity | 133.6 kJ/mol |
What Is Silicon?
It is a hard, weak translucent strong with a blue-dim metallic gloss, and is a tetravalent metalloid and semiconductor. It is an individual from bunch 14 in the occasional table: carbon is above it; and germanium, tin, lead, and flerovium are beneath it.
It is somewhat lifeless. Due to its high synthetic proclivity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first ready to plan it and portray it in an unadulterated structure. Its oxides form a group of anions called as silicates.
Its liquefying and edges of boiling over of 1414 °C and 3265 °C, separately, are the second most elevated among all the metalloids and nonmetals, being outperformed simply by boron. Silicon is the eighth most normal component known to man by mass, however seldom happens as the unadulterated component in the Earth’s hull.
It is most generally circulated in space in astronomical cleans, planetoids, and planets as different types of silicon dioxide (silica) or silicates. Over 90% of the Earth’s outside layer is made out of silicate minerals, making silicon the second most bountiful component in the Earth’s hull (around 28% by mass), after oxygen.
Uses Of Silicon
Silicon is a characteristic material, and when not already present makes some home memories of around 400 years on the planet’s oceans. Most silicon is utilized industrially without being isolated, regularly with very little handling of the normal minerals. Such use incorporates modern development with dirt, silica sand, and stone.
Uses Of Silicates
Silicates are utilized in Portland concrete for mortar and plaster and blended in with silica sand and rock to make concrete for walkways, establishments, and streets. They are likewise utilized in whiteware earthenware production like porcelain, conventional silicate-based soft drink lime glass, and numerous other specialty glasses.
Silicon Mixtures
Silicon mixtures, for example, silicon carbide are utilized as abrasives and parts of high-strength ceramics. Silicon is the premise of the broadly utilized engineered polymers called silicones.
Silicon Age
The late twentieth century to mid 21st century has been depicted as the Silicon Age (otherwise called the Digital Age or Information Age) as a result of the enormous effect that natural silicon has on the cutting edge world economy.
The little piece of profoundly decontaminated basic silicon utilized in semiconductor gadgets (<10%) is fundamental for the metal-oxide-semiconductor (MOS) semiconductors and incorporated circuit chips utilized in most present-day innovation, for example, cell phones and different PCs.
The most generally utilized silicon gadget is the MOSFET (metal-oxide-semiconductor field-impact semiconductor), which has been made in bigger numbers than some other gadget ever. Free silicon is additionally utilized in steel refining, aluminum-projecting, and fine substance enterprises (regularly to make smoldered silica).
Silicon is a fundamental component of science. Just follows are needed by creatures, however, some ocean wipes and microorganisms, like diatoms, emit skeletal constructions made of silica. Silica is kept in many plant tissues.
Summary
Silicon has atomic number 14. It is a hard, weak translucent strong with a blue-colored nucleus. It has two electrons in its first shell, eight electrons in subsequent shells, and four electrons in the third shell.
Silicates are used in Portland concrete for mortar and plaster and blended in with silica sand and rock to make concrete for walkways, establishments, and streets.
What are Silicates?
Silicate compounds are the most prevalent source of silicon. Silica has the empirical formula SiO2 and is the only stable silicon oxide. Silica isn’t a silicon atom connected to two oxygen atoms by two double bonds. Silica is made up of one silicon atom joined to four oxygen molecules via four single bonds.
Because silicon’s 3p orbitals make it more energetically favorable to forge four single bonds with each oxygen atom rather than two double bonds with each oxygen atom, silicon dioxide has this molecular shape rather than carbon dioxide’s typical shape.
-
As a result, silicates form -Si-O-Si-O- networks, which are known as silicates.
-
Because each silicate has a different net average, the empirical form of silica is SiO2.
-
The fundamental unit of silicates, the tetrahedral SiO44- complex (see Figure 3), can bind together in a variety of ways to form a variety of minerals.
Silicon is a necessary component of minerals, much as carbon is an inorganic substance.
1. Neo-Silicates
The silicate tetrahedral in nesosilicates does not share oxygen molecules with another silicate tetrahedral, instead of balancing its charge with other metals. A single tetrahedral silica unit is the fundamental structure of neo-silicate.
-
The empirical formula for a neo-silicate’s core structure is SiO44-.
-
Neo-silicates are the outermost minerals in the olivine group of minerals.
2. Soro-Silicates
Two silicates tetrahedral are found in soro-silicates. Two silicates tetrahedral come together in soro-silicates by sharing an oxygen atom at one of their corners. A pair of silica tetrahedra make up the core structure of a sorosilicate.
3. Cyclosilicates
Three or more silica tetrahedral share two corners of an oxygen atom in cyclosilicates. A cyclosilicate’s core structure is a closed ring of silica tetrahedra. A cyclosilicate complex is exemplified by the mineral beryl.
4. Inosilicates
Inosilicates are complexes where each tetrahedral shares two corners with another silica tetrahedral to make a solitary chain or three corners to make a twofold chain. The center construction of an inosilicate is either a limitless single or twofold chain of silica tetrahedral.
-
The mineral gathering pyroxenes are instances of single-chain inosilicates.
-
The mineral amphibole is an illustration of a twofold chain inosilicate.
5. Phyllosilicates
Phyllosilicates are silica edifices where each tetrahedral shares three corners and makes a sheet of silicon and oxygen. The center complex of a phyllosilicate is an endless sheet of associated silica tetrahedral. The mineral powder is an illustration of a phyllosilicate complex.
6. Tectosilicates
Tectosilicates are three-layered silicate structures. The center design of a tectosilicate is a boundless organization of associated silica tetrahedral. The mineral quartz is an illustration of a tectosilicate complex.
Albeit numerous silica buildings structure network covalent solids, quartz is an especially genuine illustration of an organization strong. Silicates in everyday offer the properties of covalent solids, and this associated exhibit of properties makes them extremely valuable in the current industry.
What Are Silanes?
Silanes are silicon-hydrogen compounds. Carbon-hydrogen intensifies the structure of the foundation of the living scene with apparently unlimited chains of hydrocarbons. With a similar valence setup and accordingly similar substance adaptability, silicon might assume a part of comparable natural significance.
Yet, silicon doesn’t assume a fundamental part in our everyday science. One chief reason underlies this. Like hydrocarbons, silanes continuously fill in size as extra silicon molecules are added. In any case, there is an extremely fast finish to this pattern.
The biggest silane has a limit of six silicon molecules. Hexasilane is the biggest conceivable silane because Si-Si bonds are not especially solid. Truth be told, silanes are fairly inclined to deterioration. Silanes are especially inclined to disintegrate using oxygen.
Silanes likewise tend to trade out their hydrogens for different components and become organosilanes. Silanes have an assortment of modern and clinical employments. In addition to other things, silanes are utilized as water anti-agents and sealants.
What Are Silicones?
Silicones are manufactured silicon compounds, they are not found in nature. At the point when explicit silanes are made to go through a particular response, they are transformed into silicone, an exceptionally extraordinary silicon complex. Silicone is a polymer and is valued for its adaptability, temperature strength, low instability, general substance obstruction, and warm soundness.
Silicone has a one-of-a-kind compound construction, yet it imparts a few center primary components to the two silicates and silanes. Silicone polymers are utilized for a colossal exhibit of things. Among various things, inserts are made from silicone.
Summary
Silica has the empirical formula SiO2 and is the only stable silicon oxide. Silicon is a necessary component of minerals, much as carbon is an inorganic substance. A single tetrahedral silica unit is the fundamental structure of a neosilicate. The center complex of a phyllosilicate is an endless sheet of associated silica tetrahedral.
What Are Silicon Halides?
Silicon tends to promptly respond with incandescent light. The overall recipe portraying this is SiX4, where X addresses any halogen. Silicon can likewise extend its valence shell, and the research facility planning of [SiF6]2-is an authoritative illustration of this.
Notwithstanding, it is impossible that silicon could make such a complex with some other halogen than fluorine since six of the bigger halogen particles can’t be in great shape around the focal silicon molecule. Silicon halides are integrated to filter silicon edifices.
Silicon halides can undoubtedly be made to surrender their silicon using explicit synthetic responses that outcome in the development of unadulterated silicon.
Applications Of Silicon
Silicon is a fundamental part of the advanced industry. Its overflow makes it even more valuable. Silicon can be found in items going from cement to microprocessors.
1. Electronics
The cutting-edge areas reception of the title Silicon Valley highlights the significance of silicon in current innovation. Unadulterated silicon, which is unadulterated silicon, has the extraordinary capacity of having the option to discretely control the number and charge of the current that goes through it.
This makes silicon assume a part of most extreme significance in gadgets like semiconductors, sunlight-based cells, coordinated circuits, microchips, and semiconductor gadgets, where such current control is a need for legitimate usage. Semiconductors represent silicon’s utilization in contemporary innovation.
2. Semiconductors
Semiconductors are extraordinary materials that have neither the electrical conductivity of a channel nor of a cover. Semiconductors lie someplace in the middle of these two classes giving them an extremely helpful property. Semiconductors can control electric flow.
They are utilized to amend, intensify, and switch electrical signals and are consequently essential parts of cutting-edge gadgets. Semiconductors can be made from an assortment of materials, however, most semiconductors are made from silicon.
![]()
Doping
Doping alludes to a cycle by which contaminations are brought into ultra unadulterated silicon, consequently changing its electrical properties and transforming it into a semiconductor. Doping transforms unadulterated silicon into a semiconductor by adding or eliminating an extremely modest quantity of electrons.
Subsequently making it neither a separator nor a guide, but a semiconductor with restricted charge conduction. Unpretentious control of unadulterated silicon cross-sections through doping produces the wide assortment of semiconductors that current electrical innovation requires.
Semiconductors are made from silicon for two crucial reasons. Silicon has the properties expected to make semiconductors, and silicon is the second most plentiful component on the planet.
3. Manufacturing Glass
Glass is one more silicon derivate that is generally used by current society. If sand, a silica store, is blended in with sodium and calcium carbonate at temperatures close to 1500 degrees Celsius, when the subsequent item cools, glass structures. Glass is an especially fascinating condition of silicon.
Glass is one of a kind since it addresses a strong non-translucent type of silicon. The tetrahedral silica components tie together, yet in no crucial example behind the holding. The final product of this novel compound design is the regularly weak normally optically straightforward material known as glass.
This silica complex can be observed practically anyplace human civilization is found. Glass can be polluted by adding compound contaminations to the basal silica structure. The expansion of even a little Fe2O3 to unadulterated silica glass gives the resultant blended glass a particular green tone.
4. Fiber Optics
Present-day fiber-optic links should transfer information using undistorted light signals over tremendous distances. To attempt this errand, fiber optic links should be made of extraordinary super high immaculateness glass. The mystery behind this super high immaculateness glass is ultra unadulterated silica.
To make fiber optic links fulfill functional guidelines, the contamination levels in the silica of these fiber optic links have been decreased to parts per billion. This degree of virtue considers the immense correspondences network that our general public has come to underestimate.
5. Ceramics
Silicon assumes an essential part in the development business. Silicon, explicitly silica, is an essential fixing in building parts like blocks, concrete, earthenware production, and tiles.
Also, silicates, particularly quartz, are thermodynamically steady. This means silicon earthenware production has high hotness resilience. This property makes silicon earthenware production particularly valuable from things going from space transport frames to motor parts.
6. Polymers
Silicone polymers address one more feature of silicon’s helpfulness. Silicone polymers are for the most part portrayed by their adaptability, protection from synthetic damage, impermeability to water, and their capacity to hold their properties at both high and low temperatures.
This variety of properties makes silicone polymers extremely valuable. Silicone polymers are utilized in protection, cookware, high-temperature greases, clinical hardware, sealants, glues, and even as an option in contrast to plastic in toys.
Formation Of Silicon
As silicon isn’t ordinarily found in its unadulterated state, silicon should be artificially extricated from its normally happening compounds. Silica is the most predominant type of normally happening silicon. Silica is an emphatically reinforced compound and it requires a decent arrangement of energy to remove the silicon out of the silica complex.
The chief method for this extraction is through a compound response at an extremely high temperature.
-
The blend of silicon is essentially a two-stage process.
-
To start with, utilize a strong heater to warm up the silica to temperatures north of 1900 degrees celsius, and second, add carbon.
-
At temperatures, more than 1900 degrees celsius, carbon will decrease the silica compound to unadulterated silicon.
Purification Of Silicon
For a few silicon applications, the purity of newly delivered silicon isn’t acceptable. To satisfy the need for high virtue silicon, methods have been contrived to additionally refine the purity of separated silicon.
Refinement of silicon includes taking orchestrated silicon, transforming it into a silicon compound that can be effortlessly refined, and afterward separating this new framed silicon compound to yield an ultra unadulterated silicon item. There are a few unmistakable cleaning strategies accessible, yet most synthetic types of filtration include both silane and silicon halide edifices.
Random Data
-
Silicon is the eighth most plentiful component known to man.
-
Silicon was recognized in 1787 yet first found as a component in 1824.
-
Silicon is a significant component in the digestion of plants, yet not vital in the digestion of creatures.
-
Silicon is innocuous to ingest and infuse into the body however it is hurtful to breathe in.
-
Silicosis is the name of the illness related to breathing in a lot of the silicon compound silica. It principally burdens development laborers.
-
Silica is a significant synthetic part of asbestos.
Summary
Silicon can likewise extend its valence shell, and the research facility planning of [SiF6]2- is an authoritative illustration of this. Silicon halides can undoubtedly be made to surrender their silicon using explicit synthetic responses that outcome in the development of unadulterated silicon. Silicon is the second most plentiful component on the planet.
History Of Silicon
Attributable to the wealth of silicon in the Earth’s hull, normal silicon-based materials have been utilized for millennia. Silicon rock precious stones were recognizable to different antiquated developments, for example, the predynastic Egyptians who involved it for dots and little containers, just as the old Chinese.
Glass containing silica was fabricated by the Egyptians since something like 1500 BC, just as by the antiquated Phoenicians. Regular silicate compounds were likewise utilized in different sorts of mortar for the development of early human abodes.
What Is Silicon Age And Why It Is Important?
The “Silicon Age” alludes to the late twentieth century to mid 21st century. This is because of silicon being the predominant material of the Silicon Age (otherwise called the Digital Age or Information Age), like how the Stone Age, Bronze Age, and Iron Age were characterized by the prevailing materials during their times of civilization.
The critical part or “workhorse” of the silicon transformation is the silicon MOSFET (metal-oxide-silicon field-impact transistor). It was the primary minimal semiconductor that could be scaled down and efficiently manufactured for a wide scope of uses.
Since then, at that point, the large-scale manufacturing of silicon MOSFETs and MOS coordinated circuit chips, alongside persistent MOSFET scaling down at an outstanding speed (as anticipated by Moore’s law), has prompted progressive changes in innovation, economy, culture, and thinking.
The MOSFET has since turned into the most broadly fabricated gadget ever, with an expected all-out of 13 trillion MOSFETs having been made between 1960 and 2018.
Worldwide Applications Of Silicon
Since silicon is a significant component in high-innovation semiconductor gadgets, many spots on the planet bear its name. For instance, Santa Clara Valley in California obtained the epithet Silicon Valley, as the component is the base material in the semiconductor business there.
From that point forward, numerous different spots have been named in basically the same manner, remembering Silicon Forest for Oregon, Silicon Hills in Austin, Texas, Silicon Slopes in Salt Lake City, Utah, Silicon Saxony in Germany, Silicon Valley in India, Silicon Border in Mexicali, Mexico, Silicon Fen in Cambridge, England, Silicon Roundabout in London.
Characteristics Of Silicon
Physical And Nuclear
Silicon solidifies in a precious stone cubic gem structure by shaping sp3 cross breed orbitals.
A silicon atom has fourteen electrons. In the ground state, they are organized in the electron setup [Ne]3s23p2. Of these, four are valence electrons, possessing the 3s orbital and two of the 3p orbitals.
Like different individuals from its gathering, the lighter carbon and the heavier germanium, tin, and lead, it has a similar number of valence electrons as valence orbitals:
-
Subsequently, it can finish its octet and acquire the steady honorable gas arrangement of argon by shaping sp3 crossover orbitals.
-
Framing tetrahedral SiX4 subsidiaries where the focal silicon molecule shares an electron pair with every one of the four particles it is fortified too.
Electrical Properties
At standard temperature and strain, silicon is a glossy semiconductor with a pale blue dim metallic brilliance; as ordinary for semiconductors, its resistivity drops as temperature rises. This emerges because silicon has a little energy hole (band hole) between its most elevated involved energy levels (the valence band) and the least empty ones (the conduction band).
The Fermi level is somewhere between the valence and conduction groups and is the energy at which a state is as liable to be involved by an electron as not. Consequently, unadulterated silicon is adequately a separator at room temperature.
In any case, doping silicon with a pnictogen like phosphorus, As, or antimony presents an additional one electron for each dopant and these may then be energized into the conduction band either thermally or photolytically, making an n-type semiconductor.
Additionally, doping silicon with a gathering of 13 components like boron, aluminum, or gallium brings about the presentation of acceptor levels that trap electrons that might be energized from the filled valence band, making a p-type semiconductor.
Isotopes Of Silicon
Normally happening silicon is made out of three stable isotopes:
1. Si-28 (92.23%)
2. Si-29 (4.67%)
3. Si-30 (3.10%).
Out of these, the main Si-29 is useful in NMR and EPR spectroscopy, as it is the just one with an atomic twist. All three are delivered in Type Ia supernovae through the oxygen-consuming cycle, with Si-28 being made as a feature of the alpha interaction and subsequently the most bountiful.
Silicon-Consuming Interaction
The combination of Si-28 with alpha particles by photodisintegration improvement in stars is known as the silicon-consuming interaction; it is the last phase of heavenly nucleosynthesis before the fast breakdown and rough blast of the star being referred to in a sort II supernova.
Twenty radioisotopes have been described, the two most stable being Si-32 with a half-life of around 150 years, and Si-31 with a half-life of 2.62 hours. All the leftover radioactive isotopes have half-experiences that are under seven seconds, and most of these have half-experiences that are short of what one 10th of a second.
![]()
Silicon As An Atomic Polymer
Silicon has one known atomic isomer, 34mSi, with a half-life under 210 nanoseconds. Si-32 goes through low-energy beta rot to P-32 and afterward stable S-32. Si-31 might be created by the neutron enactment of regular silicon and is in this way valuable for quantitative examination.
-
It tends to be handily distinguished by its trademark beta rot to stable P-31, in which the radiated electron conveys up to 1.48 MeV of energy.
-
The known isotopes of silicon range in mass number from 22 to 44.
-
The most widely recognized rot method of the isotopes with mass numbers lower than the three stable isotopes is backward beta rot, essentially shaping aluminum isotopes (13 protons) as rot products.
-
The most well-known rot mode for the heavier shaky isotopes is beta rot, principally framing phosphorus isotopes (15 protons) as rot products.
Silicon can enter the seas through groundwater and riverine transport. Huge motions of groundwater input have an isotopic organization which is particular from riverine silicon inputs. Isotopic varieties in groundwater and riverine transports add to varieties in maritime Si-30 qualities.
Presently, there are significant contrasts in the isotopic upsides of profound water on the planet’s sea bowls. Between the Atlantic and Pacific seas, there is a profound water 30Si angle of more noteworthy than 0.3 parts per thousand. Si-30 is most usually connected with usefulness in the oceans.
Science And Mixtures
C-X and Si-X bond energies (kJ/mol)[27]
| X = | C | Si | H | F | Br | I | O- |
|---|---|---|---|---|---|---|---|
| C-X | 368 | 360 | 435 | 453 | 293 | 216 | ~360 |
| Si-X | 360 | 340 | 393 | 565 | 310 | 234 | 452 |
How Does Silicon React with Temperature Difference?
Glasslike mass silicon is somewhat idle, yet turns out to be more responsive at high temperatures. Like its neighbor aluminum, silicon shapes a slender, persistent surface layer of silicon dioxide (SiO2) that shields the metal from oxidation.
Reactivity Of silicon with different fluids
Along these lines, silicon doesn’t quantifiably respond with the air under 900 °C, yet the development of the glassy dioxide quickly increments between 950 °C and 1160 °C, and when 1400 °C is reached, climatic nitrogen likewise responds to give the nitrides SiN and Si3N4. Silicon responds with vaporous sulfur at 600 °C and vaporous phosphorus at 1000 °C.
This oxide layer by the by doesn’t forestall response with the incandescent light; fluorine assaults silicon overwhelmingly at room temperature, chlorine does as such at around 300 °C, and bromine and iodine at around 500 °C.
With Fluid Acids
Silicon doesn’t respond with most fluid acids, yet is oxidized and complexed by hydrofluoric corrosive combinations containing either chlorine or nitric corrosive to shape hexafluorosilicates. It promptly breaks up in hot fluid antacid to frame silicates.
With Alkyl Halides
At high temperatures, silicon likewise responds with alkyl halides; this response might be catalyzed by the copper to straightforwardly integrate organosilicon chlorides as antecedents to silicone polymers.
After dissolving, silicon turns out to be amazingly receptive, alloying with most metals to shape silicides, and diminishing most metal oxides because the hotness of development of silicon dioxide is so huge. Accordingly, holders for fluid silicon should be made of stubborn, inert materials like zirconium dioxide or gathering 4, 5, and 6 borides.
Coordinations In Silicon
Tetrahedral Coordination
Tetrahedral coordination is a significant underlying theme in silicon science similarly for all intents and purposes for carbon science. In any case, the 3p subshell is preferably more diffuse over the 2p subshell and doesn’t hybridize so well with the 3s subshell.
Octahedral Coordination
Accordingly, the science of silicon and its heavier congeners shows huge contrasts from that of carbon, and subsequently, octahedral coordination is likewise significant. For instance, the electronegativity of silicon (1.90) is considerably less than that of carbon (2.55).
Because the valence electrons of silicon are further from the core than those of carbon and henceforth experience more modest electrostatic powers of fascination from the core.
The helpless cross-over of 3p orbitals likewise brings about a much lower inclination toward catenation (arrangement of Si-Si bonds) for silicon than for carbon, because of the attending debilitating of the Si-Si bond contrasted with the C-C bond:
-
The normal Si-Si bond energy is roughly 226 kJ/mol
-
Contrasted with a worth of 356 kJ/mol for the C-C bond.
This outcomes in increase reinforced silicon compounds, by and large, being significantly less steady than their carbon partners, an illustration of the twofold bond rule. Then again, the presence of spiral hubs in the 3p orbitals of silicon recommends the chance of hypervalent, as found in five and six-coordinate subsidiaries of silicon like SiX−5 and SiF2−6.
Lastly, given the expanding energy hole between the valence s and p orbitals as the gathering is slid, the divalent state fills in significance from carbon to lead, so a couple of temperamental divalent mixtures are known for silicon;
-
This bringing down of the principle oxidation state
-
Pair with expanding nuclear radii
-
Brings about an increment of the metallic person down the gathering.
Conductivity Of Silicon
Silicon as of now shows some beginning metallic conduct, especially in the conduct of its oxide compounds and its response with acids just as bases, and is henceforth frequently alluded to as a metalloid rather than a nonmetal. However, metallicity doesn’t turn out to be clear in bunch 14 until germanium and predominant until tin, with the developing significance of the lower +2 oxidation state.
Silicon shows clear contrasts from carbon. For instance, natural science has not had many analogies with silicon science, while silicate minerals have an underlying intricacy concealed in oxocarbons.
Greenwood and Earnshaw, pp. 328-329 Silicon will, in general, take after germanium more than it does carbon, and this similarity is improved by the d-block compression, bringing about the size of the germanium atom being a lot nearer to that of the silicon molecule than occasional patterns would predict.
Nevertheless, there are still a few distinctions due to the developing significance of the divalent state in germanium contrasted with silicon, which brings about germanium being fundamentally more metallic than silicon. bond strength brings about the shortfall of “germanone” polymers that would be undifferentiated from silicone polymers.
Summary
Silicon is the predominant material of the Silicon Age (also called the Digital Age or Information Age). The critical part or “workhorse” of the silicon transformation is the silicon MOSFET. Since silicon is a significant component in high-innovation semiconductor gadgets, many spots on the planet bear its name. A silicon atom has fourteen electrons.
Frequently Asked Questions
People usually ask following questions.
1. What is the purpose of silicon?
Silicon is one of humanity’s most useful elements. The majority of it is used to create alloys such as aluminum-silicon and ferrosilicon (iron-silicon). These are used to deoxidize steel to create dynamo and transformer [\plates, engine blocks, cylinder heads, and machine tools.
2. Is silicon hazardous to human health?
Silicon is non-toxic both as an element and in all of its natural forms, the most abundant of which are silica and silicates. Elemental silicon is a non-reactive substance that does not appear to cause fibrosis in lung tissue. Silicon may have a long-term influence on the lungs.
3. Is silicon metal or non-metal?
Silicon is a metalloid, a type of element that is neither metal nor non-metal. Silicon is neither a metal nor a non-metal; it’s a metalloid, a substance that exists in the middle of the two. According to the Chemical Heritage Foundation, Jöns Jacob Berzelius, a Swedish chemist who also discovered cerium, selenium, and thorium, first isolated silicon in 1824.
4. Is silicon a sand-based material?
However, silicon does not occur naturally in the pure form required for electronic applications, which requires fewer than one non-silicon atom in a billion. The initial material is, in fact, sand. This technique produces metallurgical grade silicon (MG-Si), which can be as pure as 99 percent pure.
5. Is silicone harmful to the skin?
They’re non-reactive, hypoallergenic, and non-comedogenic. True, silicone allergies are extremely rare, as immune cells are unable to interact directly with silicone, making it extremely safe to use on the skin. Silicone-only formulations are non-comedogenic, which means they won’t clog pores.
6. Is silicone the same as glycerin?
Glycerin is generally utilized for room-temperature applications, while silicone oil is commonly used for extreme temperature applications, especially when icing is a problem.
7. Is it possible to produce silicon?
Industrial silicon production necessitates harsh and costly conditions. Impure silicon is made by melting silica in a furnace at roughly 1,700°C and reacting it with carbon. Chemically purified silicon is melted and gently recrystallized to provide high-quality silicon for chips.
8. Is silicone a good material to use?
The verdict: Unless you’re dealing with an open wound on your face, silicones aren’t going to help your skin in any way. While silicone-filled serums and moisturizers may temporarily improve the appearance and feel of your skin, they do not contribute to its long-term health and improvement.
9. Is silicone beneficial to oily skin?
Silicones Aren’t Good For Oily, Acne-Prone Skin (But Good For Other Skin Types) Because these silicones have low volatility, they don’t truly evaporate off the skin and can form an occlusive barrier, which isn’t ideal for those who suffer from clogged pores.
10. Is silicone oil hazardous to one’s health?
Silicone vapors are normally easily tolerated, but very high amounts can result in death owing to respiratory collapse within minutes. The gases and oxidation products can be irritating and hazardous at high temperatures, and in very high concentrations, they can induce depression and death.
Conclusion
Silicon is a synthetic component having symbol Si and atomic number 14. It is a hard, weak translucent strong with a blue-colored nucleus. It has two electrons in its first shell, eight electrons in subsequent shells, and four electrons in the third shell.
Silicates are utilized in Portland concrete for mortar and plaster and blended in with silica sand and rock to make concrete for walkways, establishments, and streets.