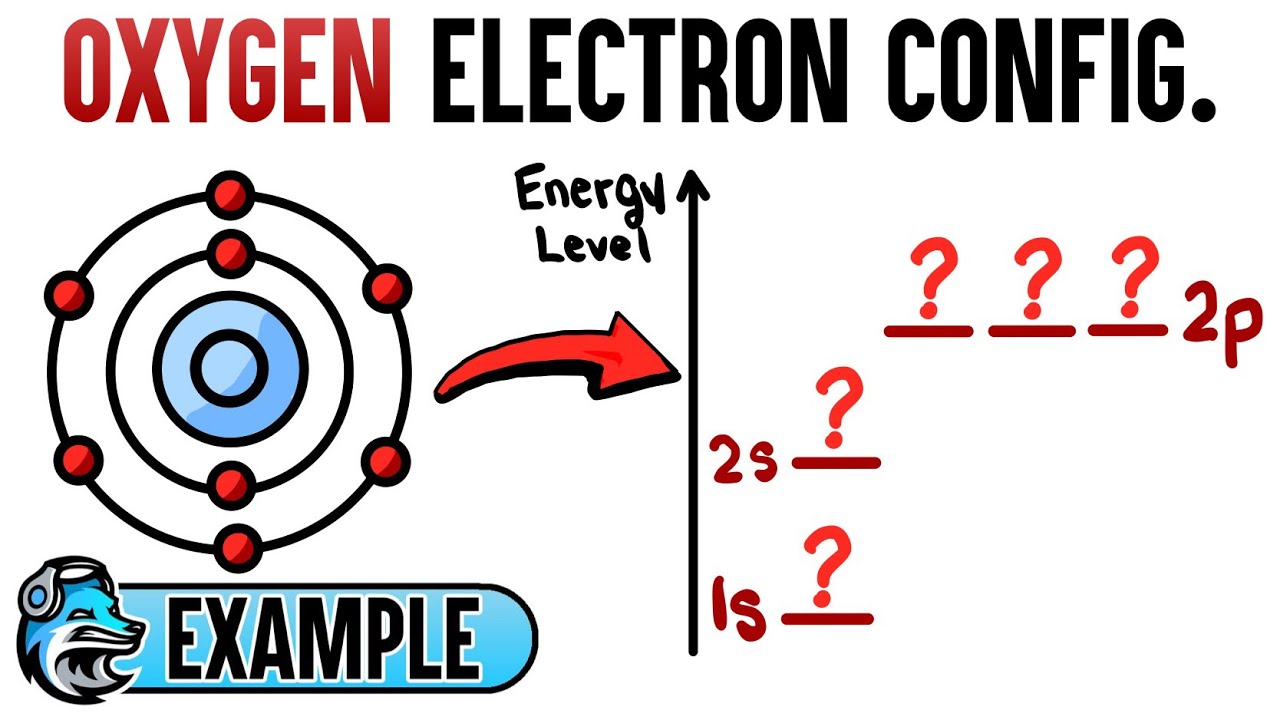
Number Of Electrons In Oxygen Are 8. In the electron configuration for oxygen, the initial two electrons reside in the 1s orbital. The remaining two electrons for O go into the 2s orbital. The four leftover electrons will be placed in the 2p orbital. As a result, the electron configuration of the O atom will be 1s22s22p4.
Characteristics Of Oxygen Atom
| 1. Atomic number | 8th |
|---|---|
| 2. Atomic weight | 15.9994 |
| 3. Melting point | 218.4 °C (361.1 °F) |
| 4. Boiling point | 183.0 °C (297.4 °F) |
| 5. Density | (1 atm, 0°C) 1.429 grams per liter |
| 6. Oxidation states | 1, 2, +2 |
| 7. Electron configuration | 1s22s22p4 |
What Is The Number Of Oxygen In Periodic Table?
Let’s have a look at the periodic table to get a solution! The periodic table provides data on every element in the universe in its neutral condition. When an atom’s net charge is zero, that is, when the number of protons equals the number of electrons, it is said to be in its neutral state.
Oxygen, with the symbol O, is the eighth element in the periodic table. This indicates that it is in its neutral state with eight electrons. It has eight protons since it is neutral! Every element in the periodic table has another number connected with it: its atomic mass. The atomic mass of oxygen is 16 AMU (Atomic Mass Units).
How Many Electrons, Protons And Neutrons Are In Oxygen Atom?
With a total of 8 electrons, oxygen is the eighth element. In the electron configuration for oxygen, the initial two electrons will be in the 1s orbital. The remaining two electrons for O go into the 2s orbital as 1s can only hold two electrons. The four leftover electrons will be placed in the 2p orbital. As a result, the electron configuration of the O atom will be 1s22s22p4.
Protons and neutrons have nearly the same mass, while electrons have extremely little mass. The electron counts for zero AMU, while protons and neutrons each count for one. So, if the atomic mass of oxygen is 16, we have 16 protons and neutrons in total. We have 8 neutrons after subtracting 8 protons from the total of 16 protons and neutrons.
What Are Isotopes?
This computation can be quite difficult! The atomic mass of an element is the average of all of the element’s isotopes. Isotopes are elements having a different number of neutrons in their atoms. The element’s chemical properties do not vary, but its atomic mass does if it contains 8 neutrons versus 9 neutrons, for example.
Because each element’s atomic mass is averaged among all stable isotopes, we sometimes encounter decimal figures for the atomic mass. Carbon, for example, has an atomic mass of 12.01 AMU (the 6th element on the periodic table). This means that most carbon atoms have six protons and six neutrons, whereas others have six protons and maybe seven or eight neutrons.
For Example
The atomic mass of most carbon atoms is extremely near to 12 AMU because they have 6 protons and 6 neutrons. Scientists have discovered the relative abundance of each element and isotope, allowing us to check up the frequency of each isotope.
Because the isotope of oxygen with 8 protons and 8 neutrons accounts for 99.7% of all oxygen atoms, it’s reasonable to assume that oxygen usually always has 8 protons and 8 neutrons.
For example, while we commonly refer to the gas we breathe as “oxygen,” it is the molecule O2 that we are inhaling (two oxygen atoms bonded together).
Number Of Electrons In Oxygen Atom
This is because oxygen has a finite quantity of electrons. Electrons arrange themselves on atoms in a specific fashion, and we can think of them as residing in “shells” around the nucleus. For the time being, we can consider atoms to have “inner” and “outer” shells.
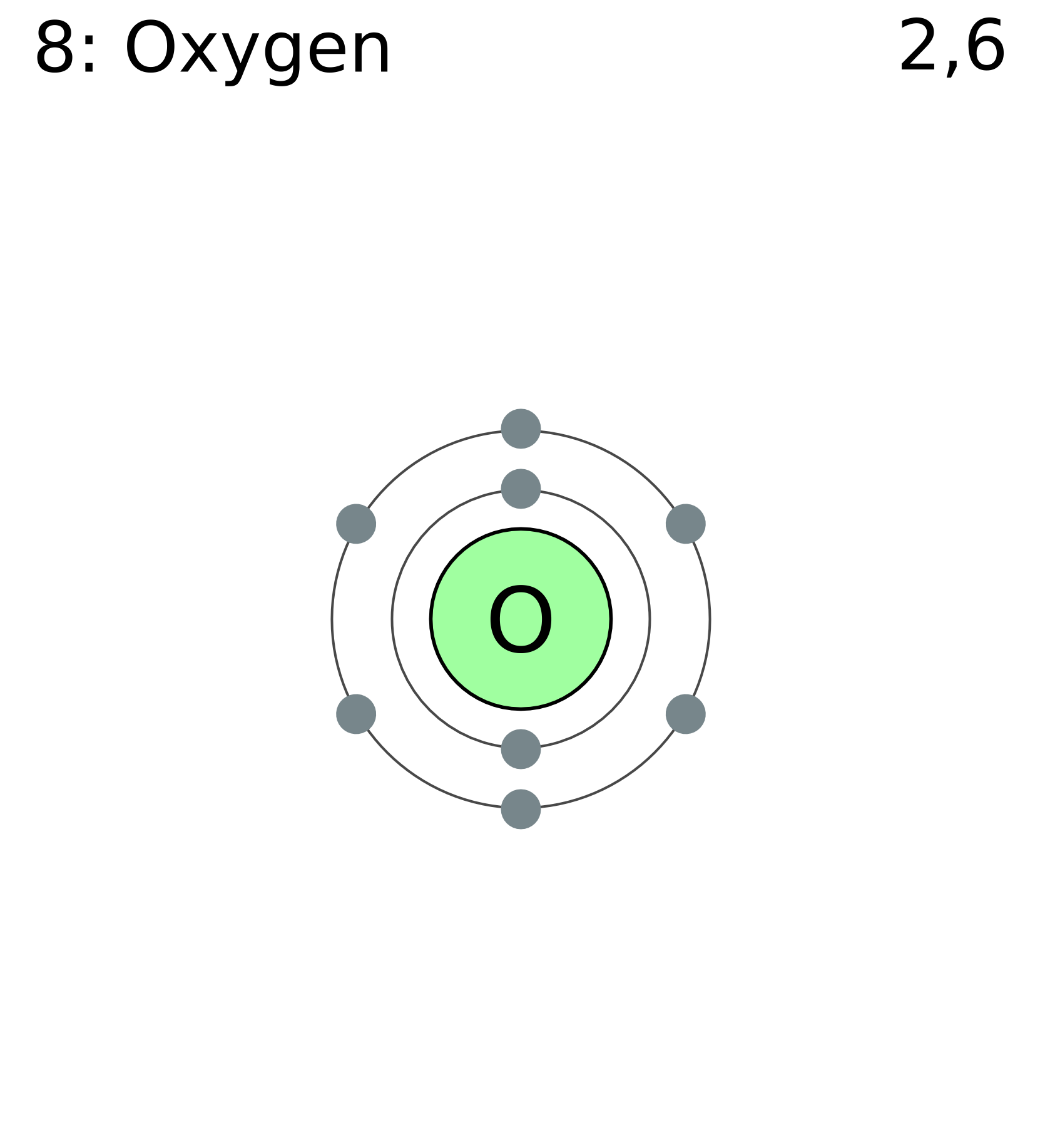
Oxygen has a two-electrons-per-shell inner shell and an eight-electrons-per-shell outer shell. Because electrons are filled from the innermost to the outermost shell, we put two in the inner shell and the remaining six in the outer shell because oxygen has eight electrons.
As we can see, there are still two empty locations in the outer shell (which can contain 8) for electrons to go, and the oxygen would like to fill those [\spots.
It accomplishes so in an ingenious way:
-
It searches for a companion!
-
When two oxygen atoms combine, they have a total of 16 electrons.
-
They each obtain half of the remaining electrons (16 total - 4 shared = 12 remaining, 12/2 = 6 each) by “sharing” four of them.
-
Two of these six go into the inner shell of each oxygen, while the other four go into the outer shell.
-
The oxygens are now moving closer together, sharing the four electrons they didn’t “keep.”
-
This gives them both the impression of having a full exterior shell (4 they contain, 4 that are shared between them).
-
This is the oxygen gas that both you and I inhale!
Characters That Are Manifested By Electron Number In An Atom
This is just one example of what can be predicted by counting electrons. The number of electrons in an element can often reveal its color, whether or not it is a magnet, and what types of other elements it will react with.
It’s also as simple as looking up the atomic number on the periodic table to see how many protons and electrons another element has (the big number at the top of the box for each element). That number indicates the number of protons and, by extension, the number of electrons in an element.
Because protons have a positive charge, each proton is balanced by one electron with a negative charge, resulting in a charge-neutral element. The situation with neutrons (which have no charge) is a little more difficult, but the number of neutrons is frequently equal to or similar to that of protons?
What Is The Number Of Electrons In the Oxygen We Breathe?
The majority of oxygen atoms have eight neutrons; however, nine or ten neutrons are possible. They’re just hard to come by. The oxygen we inhale isn’t atomically pure. This molecule has 16 protons, 16 electrons, and most usually 16 neutrons between its two atoms in its molecular form, which is two oxygen atoms connected by bonds.
What Causes Oxygen to Become An Isotope?
Oxygen becomes an isotope when it loses or gets neutrons. Oxygen isotopes are extremely rare but have practical applications. In hospitals, for example, Oxygen 15, which has seven neutrons, is used for brain imaging.
What Is Oxygen’s Atomic Mass?
An oxygen atom typically has eight protons, eight neutrons, and eight electrons. The periodic table shows that oxygen has an atomic number of 8 and an atomic weight of 15.999. The atomic number indicates the number of protons in the nucleus of the atom. This is how the atom is identified.
If the atomic number was 7, the atom would be nitrogen, which has seven protons in its nucleus. The atomic nucleus contains both protons and neutrons, however, it’s worth noting that the number of neutrons and protons isn’t always equal.
The atomic weight, which is a value that refers to the average mass of the nucleus, reveals this information (in atomic mass units or AMU). The atomic weight of all known oxygen atoms would be 16 if they all had 8 protons and 8 neutrons.
However, we know that some kinds of oxygen have fewer than 8 neutrons in their nuclei, which explains why the atomic weight is less than 16. The 8 negatively charged electrons in oxygen orbit the atomic nucleus, balancing the positive charge of the 8 protons. One proton’s positive charge exactly cancels one electron’s negative charge.
Summary
With a total of 8 electrons, oxygen is the eighth element in the periodic table. The atomic mass of oxygen is 16 AMU (Atomic Mass Units) This indicates that it is in its neutral state with eight electrons and eight protons since it is neutral. The atomic mass of most carbon atoms is very near to 12 AMU because they have 6 protons and 6 neutrons.
Different Types Of Oxygen Isotopes.
16O, 17O, and 18O are the three stable isotopes of oxygen (8O).
1. Short-lived radioactive isotopes ranging from 11O to 28O have also been identified.
2. The longest-lived radioisotope is 15O, which has a half-life of 122.24 seconds, and the shortest is 11O, which has a half-life of 1.98(22)1022 seconds (though the half-lives of the neutron-unbound 27O and 28O are still unknown).
1. Stable Isotopes
16O concentrates in the O-shell late in a massive star’s existence, 17O in the H-shell, and 18O in the He-shell.
16O Isotope As A Primary Isotope
16O, 17O, and 18O are the three stable isotopes of oxygen found in nature, with 16O being the most prevalent (99.762 percent natural abundance). The standard atomic weight fluctuates between [15.99903 and 15.99977] depending on the terrestrial source (the conventional value is 15.999).
16O has a high relative and absolute abundance because it is a major product of stellar evolution and a primordial isotope, meaning it may be produced by stars that were once entirely comprised of hydrogen.
The majority of 16O is produced in stars at the end of the helium fusion process; the triple-alpha reaction produces 12C, which captures an extra 4He to produce 16O. Additional 16O is produced during the neon burning process.

Secondary Isotopes
Both 17O and 18O are secondary isotopes, which means that seed nuclei are required for their nucleosynthesis. 17O is produced primarily through the CNO cycle’s conversion of hydrogen to helium, making it a frequent isotope in stars’ hydrogen burning zones.
The majority of 18O is created when 14N (which is abundant due to CNO burning) grabs a 4He nucleus and transforms it into 18F. This isotope decays swiftly to 18O, making it abundant in helium-rich zones of stars. To generate the heavier nucleus of Sulphur, two oxygen nuclei must undergo nuclear fusion at about a billion degrees Celsius.
Uses Of Isotopes Of Oxygen
The ratio of oxygen-18 to oxygen-16 is frequently used to infer paleoclimate changes. In the Earth’s atmosphere, the isotopic makeup of oxygen atoms is 99.759 percent 16O, 0.037 percent 17O, and 0.204 percent 18O.
-
Fresh water and polar ice on Earth contain slightly less (0.1981 percent) of the heavy isotope 18O than air (0.204 percent) or seawater (0.204 percent) because water molecules with the lighter isotope are slightly more likely to evaporate and fall as precipitation (0.1995 percent). This discrepancy allows historical ice cores to be used to study temperature patterns.
-
Solid samples (organic and inorganic) are commonly held in silver cups and analyzed using pyrolysis and mass spectrometry for oxygen isotopic ratios.
Things that should be kept in mind while accurate measurements:
For accurate measurements, researchers must prevent poor or prolonged sample storage.
-
Before the creation of the unified atomic mass unit based on 12C, oxygen was assigned an atomic mass of 16.
-
Because physicists only spoke of 16O and chemists only spoke of the naturally occurring combination of isotopes, the mass scales of the two professions differed slightly.
2. Radioisotopes
Thirteen radioisotopes have been identified, with 15O having a half-life of 122.24 seconds and 14O having a half-life of 70.606 seconds being the most stable. The remaining radioactive isotopes all have half-lives of less than 27 seconds, with the bulk having half-lives of less than 83 milliseconds (ms). 24O, for example, has a half-life of 61 milliseconds.
- For isotopes lighter than stable isotopes, the most common decay mode is + decay (to nitrogen) , while the most common mode after that is decay (to fluorine).
Oxygen-13
Oxygen-13 is an unstable oxygen isotope. It has 8 protons and electrons, as well as 5 neutrons. It has a 3/2- spin and a half-life of 8.58 milliseconds. The atomic mass of this element is 13.0248 Da. It has a decay energy of 17.765 MeV and decays to nitrogen-13 via electron capture. Fluorine-14 is its primary nuclide.
Oxygen-15
Oxygen-15 is a radioactive oxygen isotope that’s commonly utilized in positron emission tomography (PET) imaging. It can be utilized in water for PET myocardial perfusion imaging and brain imaging, among other things. It contains eight protons, seven neutrons, and eight electrons. 15.0030654 Amu is the total atomic mass.
It has a 122.24-second half-life. A cyclotron is used to produce oxygen-15 by bombarding nitrogen-14 with deuterons. When gamma rays (for example, from lightning) knock neutrons out of oxygen-16 and nitrogen-14, oxygen-15 and nitrogen-13 are generated in the atmosphere:
-
With a half-life of around two minutes, the oxygen-15 isotope decays to nitrogen-15, generating a positron.
-
The positron immediately annihilates with an electron, resulting in two 511 Kev gamma rays.
-
This gamma radiation has a half-life of two minutes after a lightning bolt; however, these low-energy gamma rays only travel around 90 meters through the air on average.
-
As the “cloud” of 15O and 13N floats by, transported by the wind.
Summary
16O, 17O, and 18O are the three stable isotopes of oxygen (8O). Short-lived radioactive isotopes ranging from 11O to 28O have been identified. The longest-lived radioisotope is 15O, which has a half-life of 122.24 seconds. There are 13 known radioisotopes of oxygen, all of which have half-lives of less than 27 seconds. 15.0030654 Amu is the total atomic mass of oxygen.
Aspects Of Occurrence And Qualities Of Oxygen
The most abundant element in Earth’s crust is oxygen, which makes up 46 percent of its mass.
-
The amount of oxygen in the atmosphere is 21% by volume and 89 percent by weight in seawater.
-
It is found in rocks as acidic (sulfates, carbonates, silicates, aluminates, and phosphates) and basic (calcium, magnesium, and iron) oxides, as well as salt like compounds generated from the acidic and basic oxides, like sulfates, carbonates, silicates, aluminates, and phosphates.
-
Despite their abundance, these solid complexes are ineffective as oxygen sources due to the high cost of separating the element from its close bonds with metal atoms.
Sources Of Oxygen
At temperatures below 183 °C (297 °F), oxygen is a pale blue liquid that solidifies at around 218 °C (361 °F). Air is 1.1 times heavier than pure oxygen. Animals and some microbes receive oxygen from the environment and return carbon dioxide, but green plants ingest carbon dioxide in the presence of sunshine and produce free oxygen through photosynthesis.
Photosynthesis is responsible for almost all of the free oxygen in the atmosphere. At 20 °C (68 °F), around 3 parts of oxygen by volume dissolve in 100 parts freshwater, somewhat less in seawater. Dissolved oxygen is used by fish and other marine animals to breathe.
Natural oxygen is made up of three stable isotopes: oxygen-16 (99.7759%), oxygen-17 (0.037%), and oxygen-18 (0.037%). (0.204 percent). Several radioactive isotopes have been created artificially. The oxygen-15 (124-second half-life) is the longest-lived and has been used to research breathing in mammals.
Allotropes Of Oxygen
The allotropic forms of oxygen are diatomic (O2) and triatomic (O3) (O3, ozone). The paramagnetic of oxygen is explained by the features of the diatomic form, which shows that six electrons link the atoms while two electrons remain unpaired. The three molecules of Ozone do not form a straight line.
1. Ozone Formation
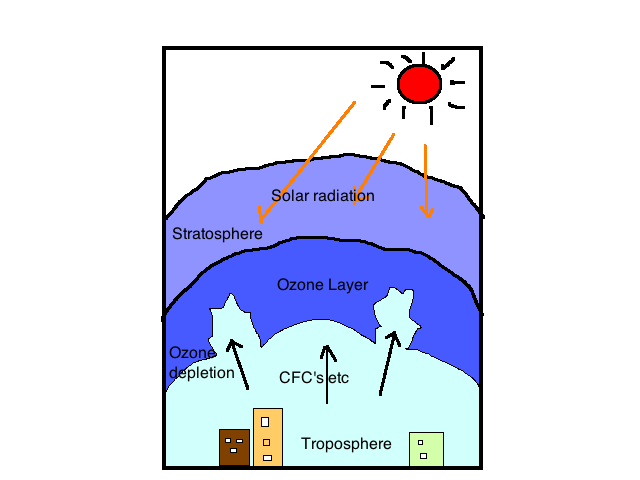
The process is endothermic (it requires energy to proceed), and the presence of transition metals or their oxides promotes the conversion of ozone back to diatomic oxygen. Pure oxygen is partially converted to ozone by a silent electrical discharge.
The reaction is also aided by the absorption of ultraviolet light with wavelengths of around 250 nanometers (nm, the nanometer, equal to 109 meters). The occurrence of this process in the upper atmosphere removes radiation that would be harmful to life on the Earth’s surface.
The unpleasant fragrance of ozone is apparent in tight locations where electrical equipment is sparking, such as generator rooms. Ozone is a pale blue gas with a density of 1.658 times that of air and a boiling point of 112 degrees Celsius (170 degrees Fahrenheit) at atmospheric pressure.
Effects of Ozone on Various Compounds
Sulfur dioxide to Sulphur trioxide, sulphones to sulfates, iodides to iodine (offering an analytical method for its measurement), and various organic compounds to oxygenated derivatives such as aldehydes and acids are all converted by ozone. The unpleasant nature of smog is due to the conversion of hydrocarbons from vehicular exhaust fumes to these acids and aldehydes by ozone.
Ozone has been utilized commercially as:
-
A chemical reagent
-
Disinfectant
-
In sewage treatment
-
Water purification
-
Textile bleaching.
Production And Consumption Of Oxygen In The Commercial Sector
When massive quantities of oxygen are required, fractional distillation of liquid air is used. Because oxygen has the greatest boiling point of the primary components of air, it is less volatile than nitrogen and argon. The method takes advantage of the fact that allowing a compressed gas to expand causes it to cool.
The following are some of the most important steps in the procedure:
1. Air is filtered to remove particulates
2. Moisture and carbon dioxide are removed by alkali absorption
3. Compressed air is compressed and the heat of compression is removed by ordinary cooling procedures
4. Compressed and cooled air is passed into coils contained in a chamber
5. Air is allowed to expand and cool in the coils
6. The expanded gas is returned to the compressor with multi-stage compression; Multiple fractionations will yield a product that is pure enough (99.5%) for most industrial applications.
In “Blowing” High Carbon Steel
It is, volatilizing carbon dioxide and other nonmetal impurities in a faster and more easily regulated process than if the air were used—the steel industry is the largest consumer of pure oxygen. The treatment of sewage using oxygen has the potential to be more efficient.
Waste incineration in closed systems employing pure oxygen has become increasingly essential. The liquid oxygen used in rocket oxidizer fuels is known as LOX, and its consumption is determined by the activities of space missions. Submarines and diving bells use pure oxygen.
Use Of Oxygen In Chemical Industry
In the chemical industry, commercial oxygen or oxygen-enriched air has replaced ordinary air in the production of oxidation-controlled products including acetylene, ethylene oxide, and methanol.
Use Of Oxygen In Medical Applications
Oxygen is used in oxygen tents, inhalators, and pediatric incubators, among other medical applications. During general anesthesia, oxygen-enriched gaseous anesthetics ensure life support.

Oxidation/Reduction
The electronegativity and electron affinity of oxygen are both high, which is typical of elements with only nonmetallic behavior. Because of the two half-filled outer orbitals, oxygen has a negative oxidation state in all of its compounds.
The oxide ion O2 is formed when these orbitals are filled by electron transport. Each oxygen in peroxides (species containing the ion O22) is believed to have a charge of 1. An oxidizing agent is defined by its ability to receive electrons via total or partial transfer. The oxidation state of such an agent is decreased when it reacts with an electron-donating molecule.
A reduction in the transition (lowering) from zero to the two states in the case of oxygen. The nomenclature used to describe oxidation and reduction is based on the behavior of oxygen, which can be considered as the “original” oxidizing agent.
Under typical conditions, oxygen forms the diatomic species O2 as well as the triatomic species ozone, O3, as discussed in the section on allotropy. There is considerable evidence for the existence of O4, a very unstable tetratomic species. There are two unpaired electrons in antibonding orbitals in the molecular diatomic form.
The presence of such electrons is confirmed by the paramagnetic behavior of oxygen. One theory for ozone’s high reactivity is because one of the three oxygen atoms is in an “atomic” state; when the O3 molecule reacts, this atom is dissociated, leaving molecular oxygen.
Reactivity Of Oxygen
A typical (ambient) temperatures and pressures, the molecular species O2 is not particularly reactive. O is a significantly more reactive atomic species. The dissociation energy (O2 2O) is high, at 117.2 kilocalories per mole.
Oxidation States Of Oxygen
In most of its compounds, oxygen has an oxidation state of 2. It can form a wide variety of covalently bonded compounds, including nonmetal oxides like:
-
Water (H2O)
-
Sulphur dioxide (SO2)
-
Carbon dioxide (CO2)
Organic compounds like:
-
Alcohols
-
Aldehydes
-
Carboxylic acids
Common acids like:
-
Sulfuric (H2SO4)
-
Carbonic (H2CO3)
-
Nitric (HNO3)
Corresponding salts like:
-
Sodium sulfate (Na2SO4)
-
Sodium Carbonate (Na (NaNO3).
The oxide ion, O2-, is found in the crystalline structure of solid metallic oxides like calcium oxide, CaO. Metallic super-oxides like potassium superoxide (KO2) have the O2- ion, but metallic peroxides like barium peroxide (BaO2) have the O22- ion.
Life’s Breath
According to the Thomas Jefferson National Accelerator Facility, oxygen is the third most abundant element in the universe. However, because of its reactivity, it was relatively uncommon in the early Earth’s atmosphere.
Cyanobacteria, which “breathe” through photosynthesis, take in carbon dioxide and exhale oxygen in the same way that modern plants do. The earliest oxygen on Earth was most likely produced by cyanobacteria, an event known as the Great Oxidation Event.
Photosynthesis by cyanobacteria was likely ongoing before significant levels of oxygen built up in Earth’s atmosphere; a March 2014 study published in the journal Nature Geoscience discovered oxides that would have required free oxygen to form in 2.95-billion-year-old rocks discovered in South Africa.
These rocks were formed in shallow oceans, implying that photosynthesis-derived oxygen first accumulated in marine habitats roughly half a billion years before it reached the atmosphere around 2.5 billion years ago.

What Would Happen If Oxygen Is Wiped Off?
Life today relies greatly on oxygen, yet its first accumulation in the atmosphere was nothing short of a tragedy. Anaerobes, or species that do not require oxygen, were wiped off by the new atmosphere. In this new world, anaerobes that we’re unable to adapt or survive in the presence of oxygen perished out.
Summary
The most abundant element in Earth’s crust is oxygen, which makes up 46 percent of its mass. Oxygen is found in rocks as acidic (sulfates, carbonates, silicates, aluminates, and phosphates) and basic (calcium, magnesium, and iron) oxides.
Ozone is a pale blue gas with a density of 1.658 times that of air and a boiling point of 112 degrees Celsius (170 degrees Fahrenheit) at atmospheric pressure.
Who’d Have Guessed?
Oxygen is a colorless gas. However, it is a pale blue liquid.
According to Carl Zorn of the Thomas Jefferson National Accelerator Facility, if you’ve ever wondered what swimming in a pool of liquid oxygen would be like, the answer is “very, very cold.” Frostbite would be a concern because oxygen must reach a temperature of minus 297.3 F (minus 183.0 C) to liquefy.
It’s dangerous to have too little oxygen. It’s the same with too much. According to the University of Florida and the business Air Products, breathing 80 percent oxygen for more than 12 hours irritates the respiratory tract and can lead to fatal fluid build-up, or edema.
Oxygen is a tough cookie to crack: An oxygen molecule (O2) can sustain pressures 19 million times higher than atmospheric pressure, according to a 2012 study published in the journal Physical Review Letters.
In 2009, on the summit of Mount Everest, the lowest amounts of oxygen ever recorded in human blood were detected. Climbers had an average arterial oxygen level of 3.28 kilopascals. When compared to the normal range of 12 to 14 kilopascals, the term “death zone” in mountaineering makes sense.
The researchers published their findings in the New England Journal of Medicine. Thank goodness for a 21 percent oxygen atmosphere. When oxygen levels hit 35% about 300 million years ago, insects were able to grow super-large: think dragonflies with hawk-like wingspans.
Currently Available Research On Oxygen
The fusion of a carbon-12 nucleus and a helium-4 nucleus produces oxygen in the hearts of stars (also known as alpha particles). However, scientists have only lately been able to see into the nucleus of oxygen and decipher its structure.
Dean Lee of North Carolina State University and his colleagues announced in March 2014 that they had figured out the nuclear structure of oxygen-16, the most common isotope of oxygen, in both its ground state (the state in which all electrons are at the lowest possible energy levels) and its first excited state (the state in which all electrons are at the highest possible energy levels).
What Difference Does Oxygen Make In Universe?
To comprehend how nuclei form in stars — from carbon to oxygen to heavier elements — is to comprehend how the universe’s fundamental building parts snap together. The nucleus of a carbon-12 molecule, with its six protons and six neutrons, is made up of three particle clusters, each with two protons and two neutrons, as discovered by Lee and his team.
Given that carbon-12 has three of these so-called alpha clusters, the researchers reasoned that oxygen-16, with eight protons and eight neutrons, would have four. The researchers were able to see how the particles in an oxygen-16 nucleus would arrange themselves using supercomputer simulations and a numerical lattice.
They discovered that there are four alpha clusters in the ground state of oxygen-16, neatly organized in a tetrahedron. “These alpha clusters are like little fuzzy spheres of these four particles, or nucleons, and these fuzzy spheres like to touch each other by some surface interaction,” Lee explained to Live Science.
But there was still one more quantum puzzle to solve. The oxygen-16 ground state and the first excited state has an uncommon property. They have the same spin, which is a value that describes how the particles revolve. They also have positive parity, which is a symmetry indicator.
Imagine inverting left and right throughout the cosmos while maintaining the same geometry of subatomic particles. Positive-parity particles would be able to view themselves as they are in this mirror reality. Negatively parity particles would have to flip-flop to avoid looking backward like a line of writing read in a mirror.
Revolutions In Oxygen
Given that the states are different, “the mystery was why the lowest two states of oxygen-16 have zero spin and positive parity,” Lee said. The simulations revealed that in its excited state, oxygen-16 rearranges its nucleus, making it look very different from the ground state.
The alpha particles assemble themselves on a square or near-square plane rather than a tetrahedral arrangement. Lee explained, “Their fundamental inherent structures were different.” The completely distinct design explains how spin and parity can remain the same even though the nuclei travel down different paths to the same destination.
Lee stated that there are still more quantum interactions and finer-grained detail to be discovered in the oxygen-16 nucleus. “There are a lot of interesting things going on inside small things like nuclei,” he explained. “And stories are being told about how they’re made that we’re finally beginning to be able to address.”
Lee’s research focuses on the origins of oxygen in the stars, while another line of oxygen research examines the element’s role in life on Earth. According to Daniel Mills, oxygen levels may have reached or exceeded today’s levels shortly after the Great Oxidation Event 2.4 billion years ago before plummeting.
FAQS
People usually ask following questions.
1. What are the top five fascinating facts about oxygen?
Oxygen-Related Facts:
-
Animals and plants both need oxygen to breathe.
-
Oxygen gas has no color, no odor, and no taste.
-
The color of liquid and solid oxygen is pale blue.
-
Oxygen is a nonmetallic element.
-
Oxygen gas is usually represented by the divalent molecule O2.
-
Combustion is aided by the presence of oxygen.
2. What is the name of a single oxygen atom?
Separating O2 (the oxygen that naturally appears in Earth’s atmosphere) into O (one atom of oxygen—otherwise known as atomic oxygen) in a lab produces atomic oxygen.
3. What is the significance of the oxygen atom?
In human bodies, oxygen atoms are a necessary component of proteins and DNA. Oxidation is the process of oxygen combining with other atoms to form compounds.
4. Is oxygen a liquid or a gas?
Oxygen is a colorless, odorless, and tasteless gas that is taken up by animals, which then convert it to carbon dioxide; plants, on the other hand, use carbon dioxide as a carbon source and return oxygen to the environment.
5. Is it possible for oxygen to exist as a solid?
At a temperature below 54.36 K (218.79 °C, 361.82 °F), solid oxygen develops at normal air pressure. Solid oxygen O2 is a transparent solid with a light sky-blue appearance due to absorption in the red region of the visible light spectrum, similar to liquid oxygen.
6. How many different oxygen allotropes are there?
Dioxygen, O2 - colorless, is one of four known allotropes of oxygen. Ozone, or O3, is a blue gas. O4 - red is called tetraoxygen.
7. Why is oxygen referred to as O2?
The distinction between oxygen (O) and oxygen (O2) is that the former is an oxygen atom, whilst the latter is a molecule made up of two O atoms bonded together. The most common form of oxygen is a diatomic gas. As a result, we refer to it as O2.
8. Is oxygen gas at what temperature?
-182.96 degrees Celsius is the boiling point of oxygen (under 1 standard atmosphere). This means that oxygen is a solid or a liquid at temperatures below that point, and a gas at temperatures above that point. So, oxygen is a liquid at -183 degrees Celsius.
9. What causes oxygen to be unstable?
Because it only contains 6 electrons in its outermost shell, one oxygen atom is unstable. To make the valence shell electrons equal to 8, the oxygen atom establishes a covalent link with another oxygen atom and shares two electrons. A stable oxygen molecule (O2) has two oxygen atoms.
10. Is oxygen a diatomic substance?
Diatomic molecules are made up of two chemically linked atoms. When two atoms are the same, such as in the oxygen molecule (O2), they form a homonuclear diatomic molecule, however when the atoms are different, such as in the carbon monoxide molecule (CO), they form a heteronuclear diatomic molecule.
11. What are the three functions of oxygen?
Production of steel, plastics, and textiles, brazing, welding, and cutting of steel and other metals, rocket propellant, oxygen therapy, and life support systems in aircraft, submarines, spaceflight, and diving are all examples of common uses of oxygen.
Conclusion
The eighth element in the periodic table is oxygen, which has a total of 8 electrons. The atomic mass of oxygen is 16 AMU (Atomic Mass Units), indicating that it is in its neutral state, containing eight electrons and eight protons.
Because most carbon atoms contain 6 protons and 6 neutrons, their atomic mass is quite close to 12 AMU. The three stable oxygen isotopes are 16O, 17O, and 18O.