Sulfur tetrafluoride has a molar mass of 108.07 g/mol. One mole of a substance’s mass (molar mass) is expressed in grams per mole (g/mol). The chemical compound SF4 stands for sulfur tetrafluoride. It transforms into a colourless corrosive gas that emits poisonous HF when it comes into touching water or moisture. Despite these negative characteristics, this molecule is required to synthesize the chemical structure of fluorine, which is employed in the pharmaceutical and specialty chemical sectors.
Structure
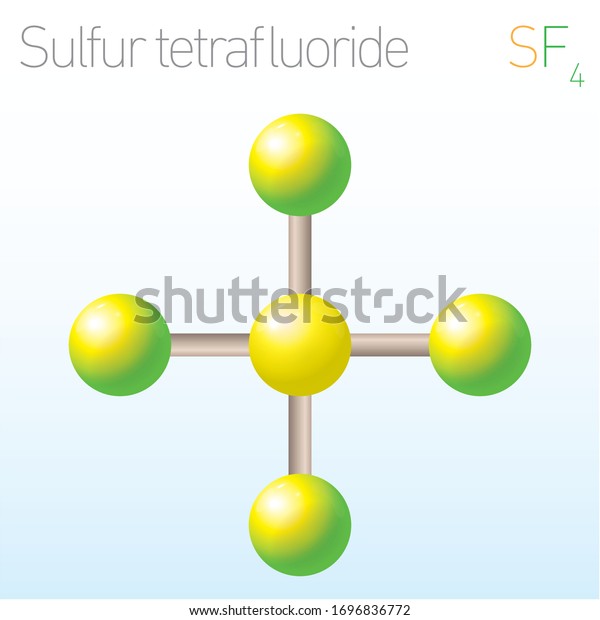
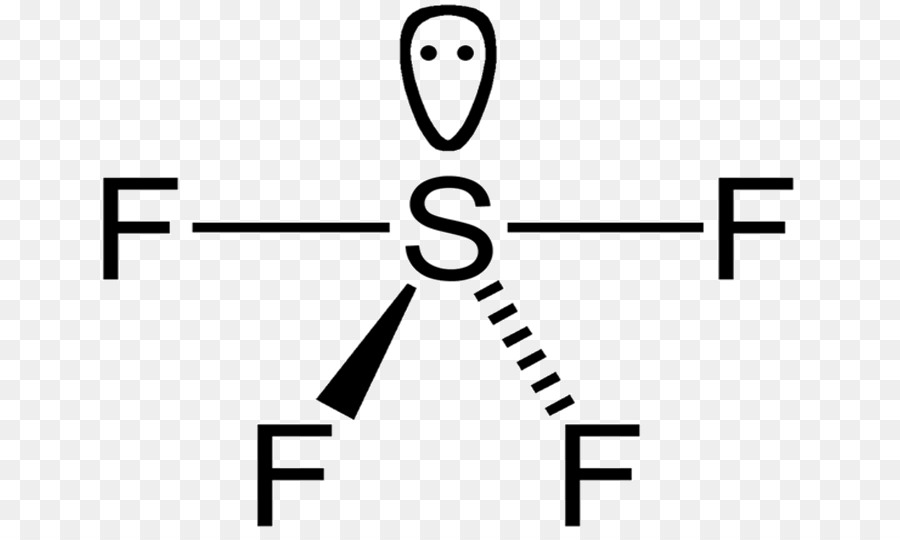
Sulfur’s formal oxidation state in SF4 is +4. Two of the sulfur’s six valence electrons form a lone pair. SF4’s structure can thus be predicted using VSEPR theory principles: it has a see-saw shape with an S in the centre. One of the three tropical locations is occupied by a nonbonding lone pair of electrons.
To conclude, there are two types of F ligands in the molecule: axial and equatorial. S–Fax = 164.3 pm and S–Feq = 154.2 pm are the relevant bond distances. The axial ligands are usually less firmly bound in hypervalent compounds. In contrast to SF4, SF6 has sulfur in the 6+ state, meaning no valence electrons remain nonbonding on sulfur.
Resulting in a highly symmetrical octahedral structure. In addition, in comparison to SF4, SF6 is chemically extremely inert. There is only one signal in the 19F NMR spectra of SF4, showing that the axial and equatorial F atom positions rapidly interconvert via pseudo rotation.
Synthesis And Production
-
The combination of SCl2 and NaF in acetonitrile produces SF4:
-
SF4 + S2Cl2 + 4 NaCl + 3 SCl2 + 4 NaF
-
SF4 can also be generated at high temperatures without a solvent.
-
In the absence of a reaction media, SF4 can also be generated with high yield utilizing sulfur (S), sodium fluoride (NaF), and chlorine (Cl2) at less-than-desirable elevated reaction temperatures (e.g., 225–450 °C).
-
Employing bromine (Br2) rather than chlorine (Cl2), S, and KF, a low temperature (e.g., 20–86 °C) technique of producing SF4 with high yield has been proven without the requirement for a reaction medium.
-
SF4 + x Br2 + 4 KBr S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr
The Use Of SF4 In Fluorocarbon Synthesis
SF4 transforms COH and C=O groups into CF and CF2 groups in chemical synthesis. The fluorocarbon equivalent to certain alcohols is widely available. Germinal difluorides are formed by ketone and aldehyde reactions. The presence of protons alpha to the carbonyl causes side reactions and a reduction in yield (30–40%).
Diols can also produce (RO) 2SO cyclic sulfite esters. Trifluoromethyl derivatives are formed when carboxylic acids are converted to trifluoromethyl derivatives. For example, at 100–130 °C, heptanoic acid is treated with SF4 to create 1, 1, 1-trifluoroheptane. Acetylene dicarboxylic acid can also be used to make hexafluoro-2-butyne.
The harmful coproducts of various fluorinations, such as unreacted SF4 and SOF2 and SO2, can be neutralized by treating them with aqueous KOH. Et2NSF3, or “DAST,” is a less hazardous replacement for SF4 in recent years. This reagent is made with the help of SF4.
- Et2NSF3 + Me3SiF + SF4 + Me3SiNEt2
Other Responses
-
Sulfur chloride pent fluoride (SF) is a kind of sulfur chloride.
-
SF4 is used to make 5Cl, an essential source of the SF5 group.
-
Sulfur dioxide is produced when SF4 is hydrolyzed.
-
SO2 + 4 HF = SF4 + 2 H2O
-
This reaction is mediated by thionyl fluoride, which does not usually interfere with the use of SF4 as a reagent.
Toxicity
-
SULFUR DIOXIDE AND HYDROGEN FLUORIDE ARE PRODUCED when SF4 combines with moisture in the lungs.
-
SO2 + 4 HF = SF4 + 2 H2O
Molar Mass And Molecular Weight
The formula weight is a quantity calculated by multiplying each element’s atomic weight (in atomic mass units) in a chemical formula by the number of atoms of that element included in the recipe, then adding the results.
Converting grams to no. of the mole is a popular request on our site. You must first determine what substance you attempt to convert to this computation. This is because the substance’s molar mass impacts the conversion. This website will show you how to calculate molar mass.

The National Institute of Standards and Technology (NIST) provided the atomic weights utilized on this webpage. The most prevalent isotopes are used. This is how isotropically weighted averages are used to derive molar mass (average molecular weight). The group of one molecule of well-defined isotopes is not the same as molecular mass.
We commonly determine the molar mass for bulk stoichiometric calculations, also known as standard atomic weight or average atomic mass. The relative weights of reagents and products in a chemical process can be determined using formula weights. Equation weights refer to the relative weights calculated from the chemical equation.
If the molecular formula is applied to calculate molar mass, the formula weight computed is the molecular weight. Divide the total weight of the atom (or group of atoms) in the formula by the formula weight and multiply by 100 to get the percentage by weight of any atom or group of atoms in a compound.
The first step in determining molar mass is to use grams per mole (g/mol) units. When we calculate a chemical compound, we find out how many grams are in one mole. The weight of all the atoms in a specific formula in atomic mass units is called the formula weight.
We can measure the molecular weight of a material by adding up the atomic weights using the compound’s chemical formula and the periodic table of elements.
Sulfur Tetrafluoride’s Mass
-
More information about each measurement unit can be found here: Sulfur Tetrafluoride molecular weight (in grams) Sulfur Tetrafluoride has the chemical formula SF4. The SI basic unit for material quantity is the mole. One mole of Sulfur Tetrafluoride is equal to 108.0586128 grams.
-
Is there a chemical molecule called sf4? The chemical compound SF4 stands for sulfur tetrafluoride. It’s an odourless, colourless gas. valence electrons of sulfur tetrafluoride: 34 electrons of valence, In each molecule of this chemical, no. of the mole of sulfur tetrafluoride are,
-
Avogadro’s number tells us that one mole of each chemical comprises 6.0221023 molecules of that component. Because you have fewer than that many sulfur hexafluoride molecules to work with, you’ll have less than one mole.
Summary
SF4 stands for Sulphur tetrafluoride. HF is formed when a colourless corrosive gas is exposed to water or moisture. In chemistry, SF4 converts COH and C=O groups to CF and CF2. Sulfur tetrafluoride mole = 108.0586128 g .The formula weight is all atoms’ total atomic mass weight in a formula. The molecular weight of a substance is calculated by summing the nuclear weights in its chemical formula.
Mole
Atoms or molecules make up all compounds. It is critical in chemistry to precisely measure the quantities of substances. The mole is utilized to express the amounts of reactants and products in chemical processes. The mole (sign mol) is the SI unit of material quantity.
There are precisely 6.022140761023 elementary entities in one mole. This is the Avogadro number fixed numerical value of the Avogadro constant, NA, as represented in the unit mol1. A system’s amount of substance, denoted by the symbol n, counts the number of defined elementary things.
An atom, a molecule, an ion, an electron, or any other particle or combination of particles can all be considered elementary entities. NA = 6.022140761023 mol1 Avogadro constant In other words, a mole is the mass of a material equal to the combined group in atomic mass units of the substance’s atoms and molecules multiplied by the Avogadro constant or Avogadro number.
A mole is a measuring unit in the International System of Units (ISU), and one of the seven base units (SI). It is represented by the symbol mol. For every mole of pure carbon-12, the mass is precisely 12 grams.

Molecular Weight
The molar mass is a physical attribute defined as a substance’s mass divided by the amount of substance in many no. of mole. Put another way, it is the mass of one mole of a specific meaning. Molar mass is measured in kilograms per mol in SI. For convenience, chemists typically always express molar masses in g/mol.
- Grams/mole = molar mass
The mass of a molecule is determined as the sum of the masses of each atom in the molecule multiplied by the number of bits of that element in the molecule (older name molecular weight). The molar mass is quantitatively identical to the molecular mass, dimensionless quantity.
Molecular and atomic mass values are given the unit dalton (Da) or unified atomic mass unit (u), which is approximately the mass of a single proton or neutron and is mathematically identical to 1 g/mol, even though they are dimensionless.
Obtaining the Molar Mass. A substance’s molar mass is computed in three steps:
-
Using the periodic table to determine elements’ atomic masses.
-
Counting the number of atoms in each of the compound’s elements.
-
Calculating the molar mass by multiplying the total of the atomic weights of the atoms that make up the compound by their numbers.
Calculate the molar mass of acetic acid, for instance.
CH₃COOH
It includes the following:
Now comes the math:
-
Carbon C = 2 = 24.0214 g/mol Carbon C = 2 = 12.0107 g/mol
-
Hydrogen H = 4.03176 g/mol = 4 1.00794 g/mol
-
Oxygen O = 2 = 31.9988 g/mol Oxygen O = 2 15.9994 g/mol Oxygen O = 2 15.9994 g/mol Oxygen O = 2 15.9994 g/mol Ox
-
Molar mass = 60.05196 g/mol = 24.0214 + 4.03176 + 31.9988 g/mol
Elements And Compounds Molar Masses
Compounds are substances made up of many atoms bonded together by chemical bonds. Compounds include, for example, the following items that can be found in every kitchen:
-
Sodium chloride (salt) NaCl
-
C12H22O11 sugar (sucrose)
-
Balsamic vinegar (acetic acid solution) CH₃COOH
The atomic mass of elements in unified atomic mass units (u) or daltons is quantitatively equal to their molar mass in grams per mole (Da). Compounds have a molar mass equal to the sum of the molar masses of the atoms that make up the compound. Water (H2O), for example, has a molar mass of roughly 1 2 + 16 = 18 g/mol.
Warning
Sulfur tetrafluoride is a caustic and unpleasant gas. Sulfur Tetrafluoride Exposure Signs and Symptoms: Skin burns, ocular and respiratory tract irritation, and pulmonary oedema may occur after acute exposure to sulfur tetrafluoride. Melena Blood in dog stool home remedies, a slow pulse rate, low blood pressure, stupor, convulsions, and cardiac arrest are all symptoms of overexposure.
Procedures for Life Support in an Emergency: Sulfur tetrafluoride poisoning can necessitate decontamination and life support for sufferers. Emergency personnel should wear protective gear depending on the type and degree of pollution. As needed, air-purifying or supplied-air respiratory equipment should be worn.

To prevent contamination, rescue trucks should carry materials such as plastic sheeting and disposable plastic bags.
-
Move victims to fresh air to avoid inhalation exposure. Emergency personnel should not inhale sulfur tetrafluoride.
-
Check vital indicators, such as pulse and respiration rate, and make a note of any injuries. If there isn’t a pulse, start CPR. Provide artificial respiration if the patient is not breathing. If breathing becomes difficult, use 100% humidified oxygen or other respiratory support.
-
Obtain authorization and further instructions for more invasive operations from the local hospital.
-
Get to a medical facility as soon as possible. Exposure to the skin or eyes:
-
Remove the victims from the situation. Sulfur tetrafluoride should not be inhaled by emergency personnel.
-
Check vital indicators, such as pulse and respiration rate, and make a note of any injuries.

You should begin CPR if you have no pulse, and you should start artificial respiration if you have no airways. Use 100% humidified oxygen or other respiratory support if breathing becomes difficult.
Remove any contaminated clothing as soon as possible. Flushing the eyes for at least 15 minutes with lukewarm water is necessary for the event of ocular exposure. 5. Use soap and water to clean all exposed skin areas thoroughly. Obtain permission from the local hospital to carry out additional invasive procedures.
Hurry to a medical facility. Ingestion Exposure: Note: Ingestion of sulfur tetrafluoride gas is not likely to constitute a significant route of exposure. 1. Evaluate vital signs, including pulse and breathing rate, and note any trauma. If there isn’t a pulse, start CPR. Provide artificial respiration if the patient is not breathing.
If breathing becomes difficult, use 100% humidified oxygen or other respiratory support. 2. DO NOT cause vomiting or seek to neutralize. 3. Give the victims water or milk: youngsters up to 1-year-old, 125 mL (4 oz. or 1/2 cup); children 1 to 12 years old, 200 mL (6 owsen or 3/4 cup); adults, 250 mL (8 oz. or 1 cup) (8 oz. or 1 cup). Water or milk should be offered only if sufferers are conscious and alert. 4. Obtain
Authorization and further instructions from the local hospital for the performance of other invasive operations. 5. Rush to a health care facility
Summary
The mole is one of the ISU’s seven base units (SI). To be classified as elementary, a particle or combination must be atomic in size. There are 6.022140761023 no. of mole—the sum of the molar masses of whole atoms in a compound.. Sulfur tetrafluoride should not be inhaled.
Computed Properties
| Property Name | Property Value |
|---|---|
| Molecular Weight | 108.06 |
| XLogP3-AA | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 107.96568382 |
| Monoisotopic Mass | 107.96568382 |
| Topological Polar Surface Area | 1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 19.1 |
| Isotope Atom Count | 0 |
## Sulfur Tetrafluoride
| Structure | 2D,3D |
|---|---|
| Chemical Safety | Compressed gas, corrosive, Acute toxic, irritant |
| Molecular Formula | F4S |
| Molar Mass | 108.07 g/mol |
| Synonyms | Tetrafluorosulfurane |
| Dates of modify | 12/11/21 |
| Create | 16/09/2004 |
Sulfur tetrafluoride is a colourless gas with a sulfur odour. When inhaled, it is highly toxic and causes severe irritation to the skin, eyes, and mucous membranes. When water and acids come into contact, they can form harmful fluoride and sulfur oxide fumes and an acidic solution.
Sulfur tetrafluoride is a heavier material than air. If containers are subjected to fire or severe heat for an extended period, they may cause a violently burst or rocket. It’s a fluoridating agent as well as an oil repellent. CAMEO Chemicals is a company that produces chemicals. A sulfur coordination entity is sulfur tetrafluoride.
Frequently Asked Questions (FAQS)
Many questions have been asked by the people
1. What is the purpose of sulfur tetrafluoride?
The gas sulfur tetrafluoride is colourless. It’s utilized as a fluorinating agent and in materials that repel water and oil. It’s also used to produce pesticides. Because it has been cited by ACGIH, DOT, NIOSH, DEP, and EPA, sulfur tetrafluoride is on the Hazardous Substance List.
2. How does sulfur tetrafluoride take its shape?
Around the central sulfur atom, there are five regions of electron density in sulfur tetrafluoride (4 bonds and one lone pair). With 102° F-S-F bond angles between the equatorial fluorine atoms and 173° between the axial fluorine atoms, these are arranged in a trigonal bipyramidal shape.
3. Is the molecule sf4 polar or nonpolar?
The molecule is polar if there are some lone pairs of electrons around the central atom and if the number is odd. SF4 is a molecule similar to this one. Only one lone pair around the central atom (Sulfur) is an odd number. As a result, SF4 is polar.
4. What is the mass of a molecule of sulfur trioxide?
More information about each measurement unit can be found here: Sulfur Trioxide molecular weight (in grams) Sulfur Trioxide has the molecular formula SO3. The mole, the SI base unit for substance quantity. One mole of Sulfur Trioxide is equal to 80.0632 grams.
5. Does sf4 have a dipole?
(c) SF4: SiF4 has no dipole moment and is tetrahedral. The molecular shape of SF4 is See-Saw. As a result, SF4 has a dipole moment.
6. What is the electrolyte strength of sulfur tetrafluoride?
Sulfur tetrafluoride is a colourless gas with a sulfur odour. It is highly poisonous when inhaled and causes severe irritation to the skin, eyes, and mucous membranes. Computed Properties (3.1) Property Value Reference Property Name XLogP3-AA 3.1 was calculated using XLogP3 3.0. (PubChem release 2019.06.18)
7. What does sf6’s Lewis structure look like?
Sulfur (S) is a Lewis structure that can hold more than eight valence electrons. It will be necessary because there are seven Fluorine (F) atoms. There are 48 valence electrons available in the Lewis structure of SF6.
8. What is the atom with the least electronegative charge?
Electronegative Elements with the Highest and Lowest Electron Negative Values Fluorine is the most electronegative element in the periodic table (3.98). In terms of polarity, caesium has the lowest possible polarity (0.79). Cesium is the most electropositive element because it is the polar opposite of electronegativity.
9. What is the bf3 structure?
The BF3 molecule has a ‘Trigonal Planar’ geometry. A model with three atoms around one atom in the middle is a ‘Trigonal Planar’ in chemistry. It’s as if they’re all peripheral atoms in one place because the 120° bond angles on each of them make them an equilateral triangle.
10. Where do you look for the official charge?
[Valence electrons on neutral atom] = Formal Charge – (number of lone electron pairs) + (12 number of bonding electrons) the group number of the periodic table corresponds to the number of valence electrons (for representative elements). Lone Pairs are the atom’s lone electrons. Each electron is worth one, so a pair is worth two.
11. How is it that sulfur can contain more than one octet?
The valence shell of an atom with more than an octet, such as phosphorus or sulfur, is said to have expanded. This happens only when the valence shell has enough orbitals to accommodate the extra electrons. The valence shell of phosphorus, for example, has a principal quantum number of n = 3.
Conclusion
Sulfur tetrafluoride has a molar mass of 108.07 g/mol. When it comes into contact with water or moisture, it turns into a colourless corrosive gas that produces toxic HF. Despite these undesirable properties, this molecule is a valuable reagent for the synthesis of part of fluorine.
To have HF, an inert gas is wet. Although flawed, the molecule aids in the part fluorine synthesis. SO4 transforms CH to CF2 and COH. Germinal difluorides result. Source of SF5. 6.0221023 mole SF COH becomes CF and CF2 Resulting difluorides.
ISU base unit mole (SI). 6.22140761023 In SI, kg/mol. Mol mass in g/mol (Da). The whole atomic molar mass.
Water has an 18 g/mole molar mass. STF inhalation is forbidden. Vital signs and respiratory rate are typical. Remember to give them 100% humidified oxygen! Ingestion of STF is improbable.

