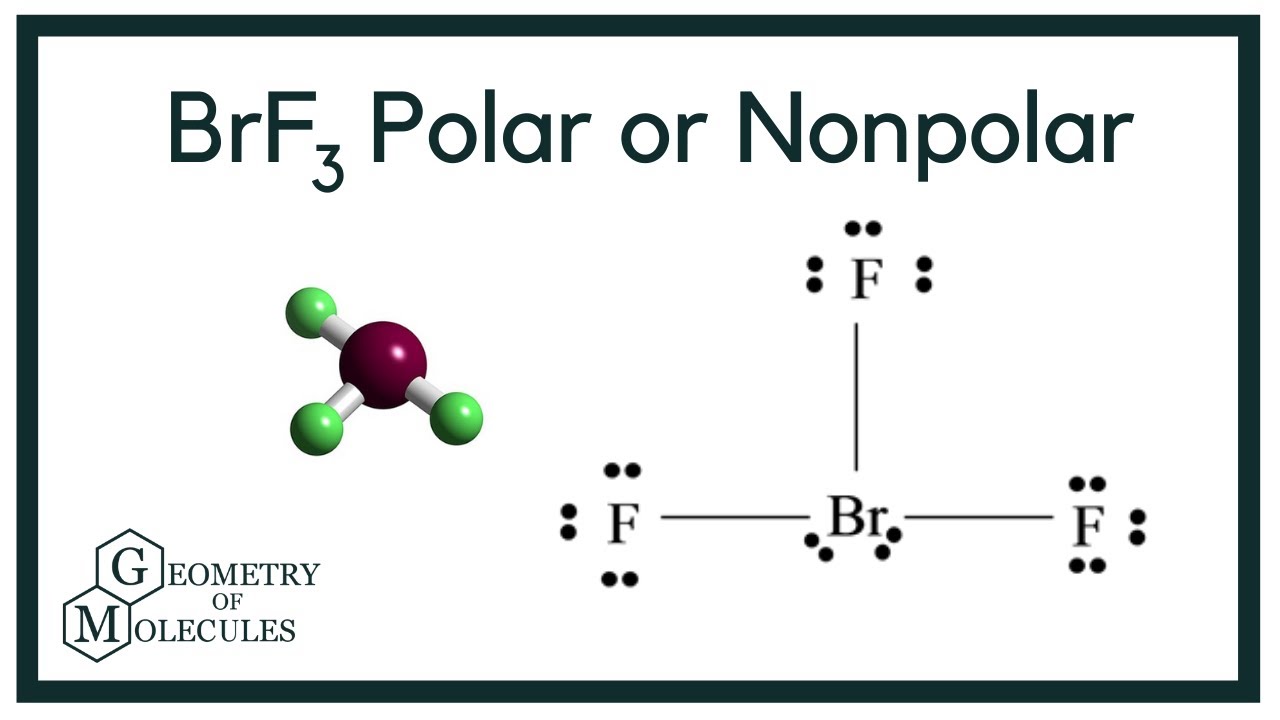
Is BrF3 a nonpolar or polar compound? BrF3 (bromine trifluoride) is a polar molecule having a twisted or distorted structure due to the two lone pairs on the bromine atom. A molecule is polar because the charge distribution of its particles is not uniform across the molecule. Bromine trifluoride is a flammable, colourless yellow liquid with a strong odour. At 48°F, it solidifies. Inhalation is highly poisonous, and metals and tissue are corroded. Containers exposed to high temperatures may violently break and rocket over an extended period.
Description
The chemical formula for brF3 is bromine trifluoride, an inorganic compound. Chemically, it’s an interhalogen. While the liquid appears to be straw-coloured, the smell is quite powerful. A common misunderstanding amongst students is whether or not bromine trifluoride is a polar compound. I’ll go into the advantages and practical uses of this question in this piece.
-
The interhalogen halogen bromine trifluoride is a halogen. It’s a viscous liquid with a pungent odour at room temperature and pressure.
-
When it comes into contact with water or another organic molecules, it reacts quickly. This is a powerful chelating agent.
-
Paul Lebeau, a well-known scientist, discovered it in 1906. At a temperature of 20°C, Bromine and fluorine were combined to form bromine Bromine trifluoride production is represented in the equation below.
-
Bromine (Br2) + fluorine (Fl2) equals 2BrF3 (bromine trifluoride)
-
This molecule’s molecular weight is 136.90 grams per mole. It is possible to arrive at the following result by applying the following formula:
-
Its mol mass is equal to one BrF3 (mol mass of Br) plus three F3 (mol mass of F) = 136.90 g/mol (mol mass of F).
-
One Bromine atom and three fluorine atoms combine to form bromine trifluoride.
-
Three fluorine atoms circle the central bromine atom. In both fluorine and Bromine, there are seven [valence electronBromines://howtodiscuss.com/t/valence-electron/141949). The outermost shells of both atoms contain seven electrons.
-
The BrF3 molecule has 28 valence electrons.
-
The bromine atom possesses two lone pairs after three fluorine atoms are covalently connected to it.
-
The fluorine atom’s electronegativity is 3.98, whereas the bromine atom’s electronegativity is 2.96. Middle-of-the-road is where the bromine atom is located due to its lower electronegative character (EC).
-
In the Br-F bond, the electronegativity difference between bromine and fluorine atoms creates polarity: the positive pole is Bromine, and the negative bar is fluorine. Bromineause of the electrical repulsion between lone pairs and bound pairs, the bromine atom contains two lone pairs, causing form distortion and the bent shape.
-
As a result, the charge distribution of its atoms is not uniform, i.e., uneven.
Manufacturing Of BF3
It is produced via the reaction of boron oxides with hydrogen fluoride, which is as follows: B2O3 + 6 HF 2 BF3 + 3 H2O. Sulfuric acid and fluorite are frequently used to produce HF on-site (CaF2).
There are various laboratory ways to solvent-free materials. The heat breakdown of BF Diazonium salts is a well-documented method.
PhF + BF3 + N2 = PhN2BF4
It can also be produced by combining sodium tetrafluoroborate, boron trioxide, and sulfuric acid.
8 BF3 + 6 NaHSO4 + 3 H2O = 6 NaBF4 + 6 B2O3 + 6 H2SO4
Polar BrF3
The polarity of boron trifluoride:
BrF3 Is A Polar Molecule (Positive Polarity).
Three fluorine atoms in bromine trifluoride surround the bromine atom.
Because of the bromine atom’s lone pairs, there is electrical repulsion.
Following the VSEPR theory, the lone pair and the bond pairs repel each other, causing the Br-F bonds to bend inward.
-
Bromine and fluorine atoms have different electronegativity, with fluorine being more electronegative than Bromine.
-
The Br-F bond’s polarity is guBromined by the electronegativity difference between bromine and fluorine atoms.
-
A polar connection’s dipole moment can never be 0 V. Each of the three Br-F has a nonzero dipole, as well.
-
An apparent passage from the Br side to the fluorine side is found in the net dipole of the molecule.
-
It is polar because of the asymmetric structure and polar Br-F bonds.
Differences
Differences between molecules that have polarity and molecules that don’t
The molecules are held together by various interatomic forces. As an example, different forces can be used in addition to ionic or covalent or hydrogen bonding or metallic bonding:
Let’s take a deeper look at the distinction between polar and nonpolar molecules.
Polar Molecules are found.
-
Dipole moment: the dipole moment of these molecules is always nonzero. Molecular polarity can be determined by its dipole.
-
When it comes to electronegativity, polar bonds are always different from one another.
-
A less electronegative atom is partially negative, whereas another is somewhat positive. In this sense, the molecule has both positive and negative poles.
-
Polar molecules have symmetrical shapes, but nonpolar molecules do not.
-
HBr and H2O are two polar compounds. You can look into why HBr is polar.
Molecules That Are Not Polar
-
In terms of dipole moment, these molecules have zero.
-
In most cases, the electronegativity of a nonpolar link created by two atoms is the same.
-
Nonpolar compounds frequently have symmetrical geometries
-
Hexane and NO2+ are examples of nonpolar compounds. You can find out why NO2 is nonpolar. +'s
Finding Polarity
When assessing the polarity of a molecule, there are several things to keep in mind.
Electronegativity
An atom’s ability to attract a bound electron pair to one side is known as electronegativity.
A covalent bond is polar if the two atoms’ electronegativity differs
Because it is nearer to the more electronegative element, the connected electron pair gains a tiny amount of negative charge.
More electronegative fluorine obtains a partial negative charge in the BrF3 molecule.
Dipole Moment
Molecule polarity can be measured using the dipole moment. The formula is as follows:
D is the product of Q and R
D = Q * R
Dipole moment (D) is the abbreviation for this property
.
The letter Q denotes atomic charges.
Symbolized by R, R denotes the distance between the two charges centres.
The sum of the charges and the distance between the positive and negative charge centres is called the total charge density.
Its SI unit is the Debye, which is represented by the letter D.
Geometrical Shape
The molecule’s geometric shape is crucial.
There are two kinds of molecules: those with an unsymmetrical structure and those with an asymmetrical one.
Bromine trifluoride has no uniform charge distribution because of its asymmetric shape.
BrF3’s characteristics
-
At room temperature, it is a straw-coloured liquid.
-
The smell is overpowering.
-
This material has a density of 2.803 g/cm3.
-
When dissolved in water, it has a melting point of 8.77° Celsius (47.79° Fahrenheit) (258.30 degrees Fahrenheit).
-
Sulphuric acid (H2SO4) rapidly dissolves it and reacts with water corrosive.
| Property Name | Property Value | Reference |
|---|---|---|
| Molecular Weight | 136.90 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| XLogP3-AA | 2.6 | Computed by XLogP3 3.0 (PubChem release 2021.05.07) |
| Hydrogen Bond Donor Count | 0 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Hydrogen Bond Acceptor Count | 3 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Rotatable Bond Count | 0 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Exact Mass | 135.91355 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Monoisotopic Mass | 135.91355 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Topological Polar Surface Area | 0 Ų | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Heavy Atom Count | 4 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 8 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
| Undefined Atom Stereocenter Count | 0 | Computed by PubChem |
| Defined Bond Stereocenter Count | 0 | Computed by PubChem |
| Undefined Bond Stereocenter Count | 0 | Computed by PubChem |
| Covalently-Bonded Unit Count | 1 | Computed by PubChem |
| Compound Is Canonicalized | Yes | Computed by PubChem (release 2021.05.07) |
BrF3’s Potential Uses
-
Uranium hexafluoride (UF6), used in nuclear fuel production, is highly valuable in its production.
-
There are numerous chemical reactions in which it is used as a fluorinating agent because of its potency.
-
Furthermore, this chemical has a high ionization potential and is also an ionizing inorganic solvent.
More Applications
Other, less well-known applications of boron trifluoride include:
Used as a flux for soldering magnesium to prepare diborane in fumigation as a dopant in ion implantation p-type dopant for epitaxially grown silicon used in sensitive neutron detectors in ionization chambers and devices to measure radiation levels in the Earth’s atmosphere.
Summary
In the case of Bromine Trifluoride, two lone pairs on its bromine atom bend its structure (BrF3). The electronegativity differences between Bromine and fluorine make the Br-F bond poBrominecause of change in the geometry and polarity of the Br-F bonds, the molecule’s charge distribution is not uniform. Therefore, Brf3 is polar in structure.
The Electron Configuration Of BrF3
BrF3 is an excellent example of an AX5 molecule since it has two lone pairs of electrons and three pairs of electrons bonded together. FLUORINE has nine electrons, while BROMINE has seven, which form three bonds with three fluorine atoms on its outer shell. The result is three pairs of bound electrons and two pairs of lone.
As stated in the VSEPR hypothesis, the molecule’s chemical structure should be trigonal pyramidal. The molecule’s structure is bent into a T-shape to reduce the repulsion between the lone pairs.
Lewis’s Platonic Form
Molecular solitary and bound atom pairs are shown in the Lewis structure, also known as the electron dot structure. Dots represent lone pairings, while lines with a bond angle represent single bonds. This will help us better comprehend compounds like BrF3’s electron pair distribution and structure.
Calculate this chemical’s valence electron number using the Lewis Structure. The line bond angle of bromine trifluoride is somewhat less than 90 degrees in a T shape. Arrange the 28 valence electrons around Br as the central element to complete the octet.
Hybridization Using BrF3
Determine how bromine trifluoride hybridizes by examining the electron configuration of the bromine atom. 1s2 2s22p6 3s23p63d104s24p5 is its abbreviation.
To establish bonds with fluorine atoms, some electrons in Bromine are moved to 4d-orbitals. FluorineBrominelarger oxidative capacity than Bromine, which causes Bromine to promote electrons to the requirBrominel. D-orbitals have enabled Bromine to hybridize.
The outermost electron shell of BrF3 includes seven electrons. In addition, it will have two lone pairs and three Br–F covalent bonds after bond formation (bonding pairs). Sp3d hybrid orbitals result from an electron pair hybridization with a hybridization value of 5. As a result, the hybridization is sp3d.
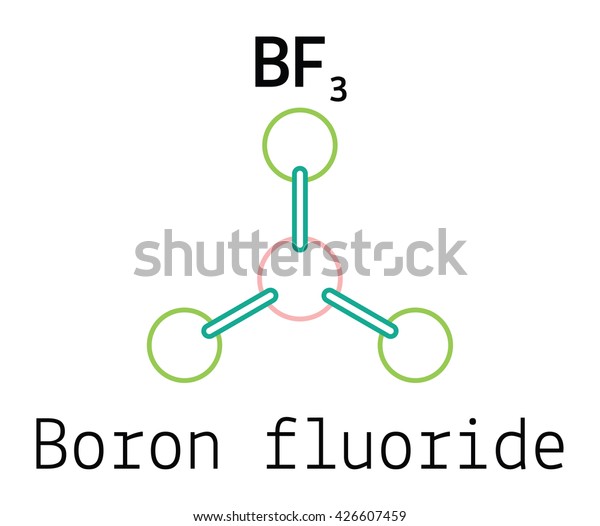
Diagram Of Molecular Orbits
The electron distribution and activity can be seen in a molecule using a molecular orbital diagram (MOD). The molecule’s physical characteristics are affected by the creation of lone pair bonds with valence electron pairs. This function determines the hybridization’s shape and where the electron is located when forming bonds.
The energy and spatial dimensions of an electron pair are crucial to the MO theory of physics. It also discusses the linear merging of atomic orbitals into molecular orbital.
The Angle Of The BrF3 Bond
With a bond angle of 86.2°, BrF3 possesses a T-shaped or trigonal bipyramidal molecular geometry (which was previously claimed). Electron pairs are more strongly opposed than Br-F bonds to form the curve. Tight bond angles can be seen in trigonal bipyramids where lone pairs are spread out more than bound teams.
Because of its sp3d hybridization in the centre of the atom, BrF3 (also known as bromine trifluoride) is a highly effective fluorinating agent. The molecule has an 86.2° bond angle and is structured like a T. Uranium hexafluoride can be made from the molecule, which is very polar. I hope so that this article has provided you with a better understanding of BrF3’s molecular structure and other properties.
Summary
BrF3 is an example of an AX5 molecule since it has two lone pairs of electrons and three pairs bonded together. BrF3’s structure is bent into a T-shape Transition moulding to reduce repulsion between lone pairs. BrF3 is a highly effective fluorinating agent. The molecule has an 86.2° bond angle. It possesses trigonal bipyramidal molecular geometry.
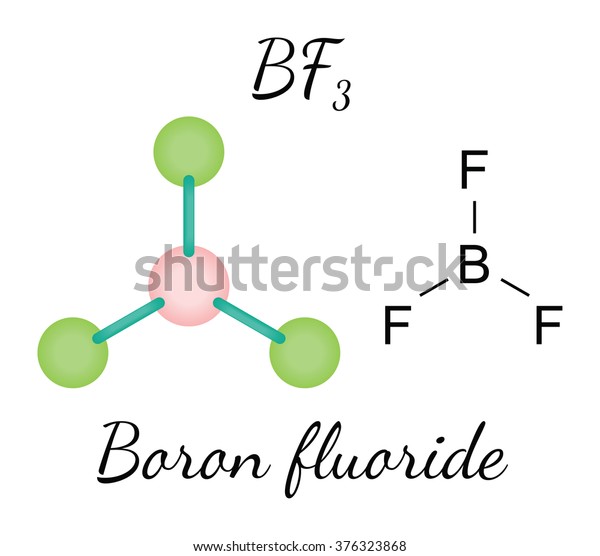
Brf3 Orientation
The fluorinating agent bromine trifluoride is a powerful interhalogen chemical. Halogens include Bromine and fluorine. Liquid forms. BrF3 is the chemical formula for this compound. At a temperature of 20 degrees Celsius, Paul Lebeau synthesized the molecule in 1906.
The molecular structure of Bromine is T-shaped, with the centre atom being Bromine. To understand its physical qualitBromineemical properties and uses, you must first grasp the molecule’s geometry, which includes hybridization, polarity, and other features.
| Name of molecule | Bromine Trifluoride |
|---|---|
| No of Valence Electrons in the molecule | 28 |
| Hybridization of BrF3 | sp3d hybridization |
| Bond Angles | 86.2 degrees |
| Molecular Geometry of BrF3 | Trigonal Bipyramidal |
Dangerous To Your Health
Inhalation irritates the upper respiratory system severely. Contact with liquids or vapours can cause serious eye burns, ulceration, and blindness. Severe burns result from contact with the skin. Mucous membranes are severely burned after ingestion. (United States Coast Guard, 1999)
The Risk Of Fire
When exposed to fire, it produces highly poisonous and unpleasant vapours. (United States Coast Guard, 1999)
Frequently Asked Questions (Faqs)
Questions that the people have asked regularly
1. Is it possible to explain the BrF3 bond in detail?
The d-orbitals of the core atom bromine are used to hybridize. Two lone pairs and three Br–F covalent bonds will be present in BrF3. Hybridization is a result of lone couplings, not groups of individuals.
2. What Intermolecular forces in the BF3 molecule?
As a result, the boron trifluoride molecule has only one intermolecular interaction: Vander Waal’s force.
3. Is there a dipole moment in BrF3?
Isn’t BrF3 a dipole, right? Is the dipole BrF3? BrF3 is a dipole, according to the original question. Bromine trifluoride (BrF3) has a molecular dipole moment of 1.19 Debye.
4. In what way are BCl3 and BF3 polar compounds, and why?
Because of BCl3’s symmetrical shape, it is a nonpolar molecule (Trigonal Planar). Boron(2.04) and chlorine(3.16) have different electronegativities, making the B-Cl bond polar, and all three B-Cl bonds are at an angle of 120 degrees to one another.
5. Is it possible for BrF3 to have dipole dipole attractions?
Bromine trifluoride (BrF3) has a dipole dipole attractions. (There must be polar bonds in polar molecules for the linked atoms to have different electronegativity. You can practice Dipole Moments by solving practice problems.
6. What is the chemical structure of BF3?
A hybrid version of BF3 has been created (Boron Trifluoride)
- You can call it Boron Trifluoride, or you can call it BF3.
- BF3 is the atomic formula of Boron Trifluoride.
- Sp2 is the most common type of hybridization.
- One hundred and twenty-degree bond angle
- Planar Trigonometry’s Geometry
7. Is it essential to know the distinction between the two types of hydrocarbons?
Because NH3 has a single pair of electrons on the nitrogen atom, electrons can only flow in one direction, making it a polar compound with a dipole moment that is greater than zero.
8. Is BF3 makes covalent bond? If it is, then why?
As the name suggests, the Boron-fluorine sp2 hybrid is the basis of the BF3 molecule. The covalent bond demonstrates that electrons are shared instead of Boron losing and fluorine gaining in the periodic table of elements. This bond was formed because of Boron’s high ionization energy.
9. Is BF3 water-soluble?
Yes, most of Earth’s hydrosphere and the bodily fluids of all known living species are made up of water, an inorganic, tasteless, odourless, and nearly colourless chemical liquid. An unknown form of life can exist without it, despite its lack of caloric or organic components.
10. Battlefield 3’s trigonal planar is of what significance?
If you combine Boron with F, you will only have three pairs of electrons circling the boron atom. According to the idea of repulsion, these three e- pairs should form an equilateral triangle (bond angles of 120 degrees). Planar triangular, as a result, BF3 is.
11. What distinguishes BF3 as covalent from ionic?
Consequently, atoms will seek as many covalent bonds as they can. There can only be three bonds in BF3 because Boron can only share three electrons. Inquiring minds may wonder why Boron and fluorine do not form ionic bonds with each other.
12. Whether or not Battlefield 3 is a “volatile” game is debated.
Yes, battlefield three is volatile. Regardless of the surrounding temperature, this liquid is highly volatile.
13. Is BF3 classified as either an acid or a basic?
Boron trifluoride (BF3) and an essential ion that can donate an electron pair combine to generate a Lewis acid. The following is an example of this type of response. The acid, in this case, is BF3, while the base is F-. The boron octet is completed by this acid-base reaction (which is electron-deficient in BF3).
14. Is BF3 capable of being hydrolyzed?
BF3 does not entirely hydrolyze like other Boron halides. Instead, it forms boric acid and fluoroboric acid after incomplete hydrolysis. Because the HF is generated first, it reacts with H3BO3.
15. Water hydrolyzes which of the following, and why BF3 BCl3 bbr3?
OH2, whereas bcl3 and bbr3 are hydrolyzed to boric acid and HCl or hbr, respectively. Because of the significant p-p back bonding in bf3, the b-f connection is solid.
Conclusion
The two lone pairs on the Bromine atom twist or distort BrF3 (bromine trifluoride). A polar molecule has a non-uniform charge distribution. Bromine and fluorine electronegativity gaps assure polarity of the Br-F bond. Molecules are held together by inter nonpolar molecules lack symmetry.
Negative charge centers add up to a positive charge Brf3 is polar due to Br-F bond shape and polarity alterations. Observe how bromine trifluoride hybridizes. BrF3 has a trigonal bipyramidal geometry (which was previously claimed). 5 electron pair hybridization gives Sp3d hybrid orbitals.
The fluorinating agent BrF3 (bromine trifluoride) 86.2° bond angle and T-shape. Trigonal bipyramidal molecular geometry.