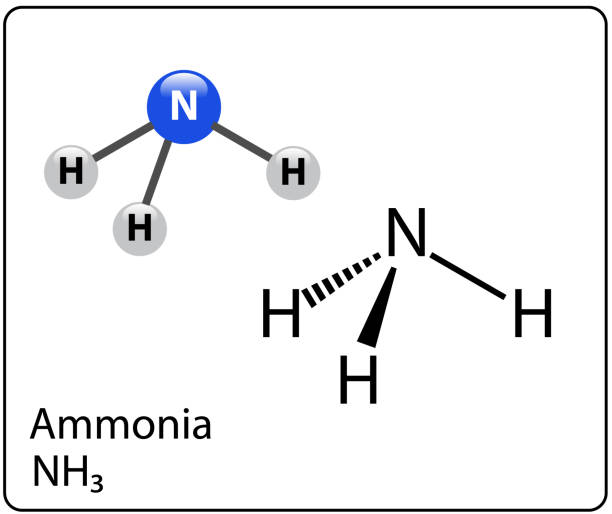
Is NH3 polar? Yes, NH3 does have a polar covalent bond. Nitrogen forms a molecule by forming a covalent link with three other atoms. Because the N-H bond and the NH3 compound are both polar, even in their gaseous condition.
Polarity or Nonpolarity of NH3 (Ammonia)
![]() NH3, often known as ammonia, contains polar molecules. When two atoms form chemical bonds, they become asymmetrical molecules with polar bonds.
NH3, often known as ammonia, contains polar molecules. When two atoms form chemical bonds, they become asymmetrical molecules with polar bonds.
![]() Ammonia, often known as NH3, is a colourless gas that is a stable hydride composed of one nitrogen atom and three hydrogen atoms. As a cleaning chemical and nitrogenous substance, ammonia has a harsh odour and a fertiliser component.
Ammonia, often known as NH3, is a colourless gas that is a stable hydride composed of one nitrogen atom and three hydrogen atoms. As a cleaning chemical and nitrogenous substance, ammonia has a harsh odour and a fertiliser component.
![]() Vegetable materials and nitrogenous animal waste products are sources of this polar molecule.
Vegetable materials and nitrogenous animal waste products are sources of this polar molecule.
![]() During rain, however, modest amounts of ammonia and ammonium salts are discovered.
During rain, however, modest amounts of ammonia and ammonium salts are discovered.
Summary
Ammonia (NH3) is a colourless gas that is a stable hydride comprised of one nitrogen atom and three hydrogen atoms. Asymmetrical molecules with polar bonds are generated when two atoms form chemical connections. Ammonia has a strong odour and serves as a fertiliser in addition to being a cleaning agent and nitrogenous material.
NH3 Molecular Structure
![]() Using Lewis structure, we will comprehend electron geometry, polarity, and other features of both polar and non-polar molecules.
Using Lewis structure, we will comprehend electron geometry, polarity, and other features of both polar and non-polar molecules.
![]() The shape of the NH3 molecules is created by the bonding pairs of electrons, or those that form bonds.
The shape of the NH3 molecules is created by the bonding pairs of electrons, or those that form bonds.
![]() Before we can determine the structure of ammonia molecules, we must first determine the valence electrons that form dipole moments.
Before we can determine the structure of ammonia molecules, we must first determine the valence electrons that form dipole moments.
![]() Nitrogen atoms have five valence electrons in their outer shell, whereas hydrogen atoms only have one, yet in this group, we have three with a central atom.
Nitrogen atoms have five valence electrons in their outer shell, whereas hydrogen atoms only have one, yet in this group, we have three with a central atom.
![]() As a result, we multiply it by three, giving us a total of eight valence atoms.
As a result, we multiply it by three, giving us a total of eight valence atoms.
![]() The overall form of NH3 is a triangular pyramidal shape because we now know the whole valence electron sets the net dipole moment.
The overall form of NH3 is a triangular pyramidal shape because we now know the whole valence electron sets the net dipole moment.
![]() A nitrogen atom serves as the core atom in the trigonal pyramidal structure, which features an asymmetrical charge distribution with three bonds and a lone pair.
A nitrogen atom serves as the core atom in the trigonal pyramidal structure, which features an asymmetrical charge distribution with three bonds and a lone pair.
| Name of molecule | Ammonia (NH3) |
|---|---|
| Bond Angles | 107.3 degrees |
| Molecular Geometry of NH3 | Pyramidal planar |
| Hybridization of NH3 | SP³ hybridization |
| NH3 oxidation number | zero |
| Is NH3 polar? | Polar |
Summary
The bonding pairs of electrons, or those that make bonds, shape the NH3 molecules. Using Lewis structure, we will understand electron geometry, polarity, and other properties of both polar and non-polar compounds. We must first determine the valence electrons that form dipole moments before we can establish the structure of ammonia molecules. Nitrogen atoms contain five valence electrons in their outer shell, whereas hydrogen atoms only have one. However, there are three with a centre atom in this group.
What are Polar and Nonpolar Molecules?
![]() Chemical compounds use many sorts of bonds to join their atoms to form molecules. Ionic, hydrogen, covalent, and metallic molecules are formed by the formation of various types of bonds.
Chemical compounds use many sorts of bonds to join their atoms to form molecules. Ionic, hydrogen, covalent, and metallic molecules are formed by the formation of various types of bonds.
![]() Ionic and covalent bonds are the most prevalent and strongest types of bonding.
Ionic and covalent bonds are the most prevalent and strongest types of bonding.
![]() Ionic bond:
Ionic bond:
![]() When two atoms with opposite charges combine to form a molecule, these bonds develop.
When two atoms with opposite charges combine to form a molecule, these bonds develop.
![]() In this case, two oppositely charged atoms stabilise each other. These bonds are typically used when there is a significant difference in the electronegativities of two atoms. In such bonds, electrons are completely transferred.
In this case, two oppositely charged atoms stabilise each other. These bonds are typically used when there is a significant difference in the electronegativities of two atoms. In such bonds, electrons are completely transferred.
![]() Covalent bond
Covalent bond
![]() These bonds are established when two or more atoms share electrons in order to stabilise one another.
These bonds are established when two or more atoms share electrons in order to stabilise one another.
![]() These bonds can be single, double, or triple depending on the number of electrons involved. These bonds may be polar or nonpolar.
These bonds can be single, double, or triple depending on the number of electrons involved. These bonds may be polar or nonpolar.
![]() When two atoms form a covalent connection, the electron density of those atoms changes. When the charge distribution of two atoms forming a bond is uneven, the bond is said to be polar.
When two atoms form a covalent connection, the electron density of those atoms changes. When the charge distribution of two atoms forming a bond is uneven, the bond is said to be polar.
![]() In such a circumstance, one of the atoms’ partial ionic charges increases.
In such a circumstance, one of the atoms’ partial ionic charges increases.
![]() This occurs when there is a big difference in electronegativity between the two atoms.
This occurs when there is a big difference in electronegativity between the two atoms.
![]() As a result, a partial ionic charge is formed, with one atom charged extremely negatively and the other charged highly positively.
As a result, a partial ionic charge is formed, with one atom charged extremely negatively and the other charged highly positively.
![]() When two atoms create a covalent connection with symmetry and an equal ionic charge on both atoms, the resulting molecule is known as a nonpolar molecule.
When two atoms create a covalent connection with symmetry and an equal ionic charge on both atoms, the resulting molecule is known as a nonpolar molecule.
![]() A non-polar connection is created when the electronegativities of both atoms are the same.
A non-polar connection is created when the electronegativities of both atoms are the same.
Summary
Many different types of bonds are used to connect the atoms in chemical compounds. The most common and powerful types of bonding are ionic and covalent connections. When there is a considerable variation in the electronegativities of two atoms, these bonds are utilised. Depending on the number of electrons involved, the bonds can be single, double, or triple. The electron density of two atoms varies when they establish a covalent bond.
Why is NH3 a polar molecule?
![]() NH3 is a polar molecule because it has three dipoles due to three bonds, and these dipoles do not cancel each other out. They combine to generate a net dipole moment.
NH3 is a polar molecule because it has three dipoles due to three bonds, and these dipoles do not cancel each other out. They combine to generate a net dipole moment.
![]() In ammonia molecules, three hydrogen atoms establish a covalent connection by sharing three electrons between the nitrogen and hydrogen atoms, leaving one lone pair on the nitrogen atom.
In ammonia molecules, three hydrogen atoms establish a covalent connection by sharing three electrons between the nitrogen and hydrogen atoms, leaving one lone pair on the nitrogen atom.
![]() According to VSEPR theory, the lone pair on the nitrogen atom exerts outward pressure on the bond, causing the NH3 form to become asymmetrical. This force on the bonds is caused by lone pair-bond pair repulsion.
According to VSEPR theory, the lone pair on the nitrogen atom exerts outward pressure on the bond, causing the NH3 form to become asymmetrical. This force on the bonds is caused by lone pair-bond pair repulsion.
![]() Nitrogen’s predicted electronegativity is 3.04, while hydrogen is 2.2. As a result of the disparity in electronegativities, the three N-H bonds produce three dipole moments in one direction.
Nitrogen’s predicted electronegativity is 3.04, while hydrogen is 2.2. As a result of the disparity in electronegativities, the three N-H bonds produce three dipole moments in one direction.
![]() The net dipole moment formed by the three dipoles in one direction determines the NH3 polar molecule.
The net dipole moment formed by the three dipoles in one direction determines the NH3 polar molecule.
![]() Nitrogen, being more electronegative, pulls the electron pair slightly towards itself in the N-H bond, causing it to become somewhat negatively charged.
Nitrogen, being more electronegative, pulls the electron pair slightly towards itself in the N-H bond, causing it to become somewhat negatively charged.
![]() Ammonia gas is extremely soluble in water, creating ammonium ions, and polar molecules combine more easily with other polar molecules.
Ammonia gas is extremely soluble in water, creating ammonium ions, and polar molecules combine more easily with other polar molecules.
![]() Water, as we know, is also a polar molecule. As a result, ammonia and water attract each other and mix easily.
Water, as we know, is also a polar molecule. As a result, ammonia and water attract each other and mix easily.
![]() It is critical to understand that, in addition to this polarity factor, they also have an additional attraction booster known as hydrogen bonding.
It is critical to understand that, in addition to this polarity factor, they also have an additional attraction booster known as hydrogen bonding.
Summary
Three hydrogen atoms form a covalent bond in ammonia molecules by sharing three electrons between the nitrogen and hydrogen atoms, leaving one lone pair on the nitrogen atom. Because it has three dipoles due to three bonds, NH3 is a polar molecule. These dipoles do not cancel each other out. The three N-H bonds produce three dipole moments in one direction due to the difference in electronegativities.
NH3 Molecular Structure
![]() As previously discussed, ammonia forms three bonds with hydrogen atoms, leaving a single lone pair on the nitrogen atom.
As previously discussed, ammonia forms three bonds with hydrogen atoms, leaving a single lone pair on the nitrogen atom.
![]() According to the VSEPR theory, the lone pair repels the three bond pairs N-H.
According to the VSEPR theory, the lone pair repels the three bond pairs N-H.
![]() The overall shape of the NH3 molecule is trigono-pyramidal. If we explain the atom positions, nitrogen is a central atom with an asymmetric charge distribution, three bonds, and one lone pair.
The overall shape of the NH3 molecule is trigono-pyramidal. If we explain the atom positions, nitrogen is a central atom with an asymmetric charge distribution, three bonds, and one lone pair.
![]() These N-H bonds have a tetrahedral arrangement. The N-H bond angle in the NH3 molecule is approximately 106.7 degrees.
These N-H bonds have a tetrahedral arrangement. The N-H bond angle in the NH3 molecule is approximately 106.7 degrees.
![]() The ammonia molecule’s hybridization is sp3.
The ammonia molecule’s hybridization is sp3.
![]() For a better understanding, the Lewis structure of the ammonia molecule is shown below. You should also read the essay on NH3 Lewis Structure, Molecular Geometry, and Hybridization.
For a better understanding, the Lewis structure of the ammonia molecule is shown below. You should also read the essay on NH3 Lewis Structure, Molecular Geometry, and Hybridization.
Summary
According to the VSEPR theory, the lone pair repels the three bond pairs N-H. The NH3 molecule has a trigono-pyramidal overall shape.
Factors That Determine NH3 Polarity
The following are the factors that determine the polarity of NH3
Geometry
![]() As previously stated, ammonia’s molecular geometry is tetrahedral. The nonpolar molecule is symmetrically distributed all throughout the nitrogen base.
As previously stated, ammonia’s molecular geometry is tetrahedral. The nonpolar molecule is symmetrically distributed all throughout the nitrogen base.
![]() The NH3 trigonal pyramidal molecular geometry was produced by two nonpolar molecules.
The NH3 trigonal pyramidal molecular geometry was produced by two nonpolar molecules.
![]() The deformed shape is due to the lone pair of electrons exerting repulsive pressure on the bonding pairs. Although the bond angle for trigonal pyramidal molecular geometry should be 109.5 degrees, it was reduced to 107 degrees due to the lone pair on the nitrogen atom.
The deformed shape is due to the lone pair of electrons exerting repulsive pressure on the bonding pairs. Although the bond angle for trigonal pyramidal molecular geometry should be 109.5 degrees, it was reduced to 107 degrees due to the lone pair on the nitrogen atom.
Electronegativity
Once uneven charges are distributed, the electronegativity difference is the initial defining characteristic of a covalent molecule. The greater the polarity, the more electronegative the atoms in these covalent bonds are, much as with nitrogen atoms.
Dipole Moment
![]() The dipole moment of a molecule is calculated as the product of the charge over the atoms and the distance between them.
The dipole moment of a molecule is calculated as the product of the charge over the atoms and the distance between them.
![]() The NH bond’s dipole moment will be from H to N. Three NH bonds will have a net dipole moment of 1.4 D. As we all know, 1 D = 3.335641034 C m.
The NH bond’s dipole moment will be from H to N. Three NH bonds will have a net dipole moment of 1.4 D. As we all know, 1 D = 3.335641034 C m.
![]() In NH3, dipole moments are computed at around 1.46D due to the polar molecule’s asymmetrical structure. These chemical substances form polar bonds to join their atoms and form asymmetrical molecules.
In NH3, dipole moments are computed at around 1.46D due to the polar molecule’s asymmetrical structure. These chemical substances form polar bonds to join their atoms and form asymmetrical molecules.
Summary
The molecular geometry of ammonia is tetrahedral. A molecule’s dipole moment is computed by multiplying the charge over the atoms by the distance between them. These chemicals form polar bonds to connect their atoms and produce asymmetrical molecules. Because of the asymmetrical shape of the polar molecule, dipole moments in NH3 are computed at around 1.46D.
Frequently Asked Questions
The following are some frequently asked questions related to is NH3 polar.
1. Is NH3 ionic or polar?
Ammonia molecules are arranged in a trigonal pyramid, with the nitrogen atom at the top and the hydrogen atoms connected at right angles. Taking both the strength of the electronegativity between bonds and the molecule’s structure into account, NH3 is identified as polar.
2. Why is NH3 classified as polar?
The charge is not equally spread over the shape due to the lone pair on the nitrogen as well as the nitrogen dragging the electrons of the N-H bonds towards itself, making the molecule polar. Ammonia has three pairs of electrons in covalent bonds and one lone pair of electrons.
3. Is NH3 soluble in water?
Ammonia gas dissolves readily in water. The hydrogen bonding that occurs between ammonia and water molecules accounts for the unusually high solubility.
4. How many polar bonds does NH3 have?
NH3 is a polar gas. Despite the fact that the electron pairs are organised tetrahedrally, only three valence electrons are bonding electrons, forming three covalent connections with hydrogen atoms. The other two are a lone pair that is closer to the molecule’s nitrogen atom. As a result of this, the molecule suffers from electron pair repulsion and asymmetry.
5. Is BrF3 polar or nonpolar?
BrF3 has a strong odour and the appearance of a straw-colored liquid. In nature, is BrF3 a polar or nonpolar molecule? BrF3 (bromine trifluoride) is a polar molecule because the presence of two lone pairs on the bromine atom causes the molecule’s shape to be twisted or bent.
6. Is NCl3 trigonal planar?
NCl3 has a trigonal pyramidal molecular shape and a tetrahedral electron geometry. The NCl3 lewis dot structure has one lone pair and three bound pairs. Nitrogen trichloride has a net dipole moment of 0.6 D.
7. Why is NH3 miscible in water?
This is due to the fact that ammonia’s hydrogen atoms are bonded with a highly electronegative nitrogen atom, whereas water molecules’ hydrogen atoms are bonded with a highly electronegative oxygen atom. Ammonia is very soluble in water due to the strong forces of attraction between ammonia and water molecules.
8. Why is NH3 more soluble?
Actually, nitrogen is a non-polar molecule N2, but in ammonia NH3, there is a large difference in the electronegativities of the N and H atoms, resulting in polarity in the ammonia molecule, and because water is a polar solvent, ammonia dissolves more easily whereas nitrogen does not.
9. Why ammonia NH3 is more soluble in water than nitrogen n2)?
Actually, nitrogen is a non-polar molecule N2, but in ammonia NH3, there is a large difference in the electronegativities of the N and H atoms, resulting in polarity in the ammonia molecule, and because water is a polar solvent, ammonia dissolves more easily whereas nitrogen does not.
10. Is NH3 hydrophilic?
As a result of this interaction, water and ammonia can create hydrogen bonds as a result. More specifically, nitrogen’s lone pair of electrons will be attracted to one of water’s partial positive hydrogen atoms. As a result of being a polar molecule, ammonia is hydrophilic, or water loving.
Conclusion
Ammonia has a tetrahedral geometrical structure because it is an asymmetrical molecule with three hydrogen atoms and one nitrogen atom. When two atoms make chemical bonds, they generate asymmetrical polar molecules. Is NH3 polar?
The answer is yes. Because of the enormous difference in electronegativity between nitrogen and hydrogen, the nitrogen-hydrogen bond is polar. The dipole moments of the N-H bond add up to a net dipole moment for the ammonia molecule, converting it into a polar molecule. Furthermore, the partial charges are not spread uniformly around the molecule, with a negative charge at the top.
Related Articles
Nh3 Lewis Structure?
CH2F2 Polar or Non Polar
Is Seh2 Polar Or Nonpolar
Bf4 Polar Or Nonpolar