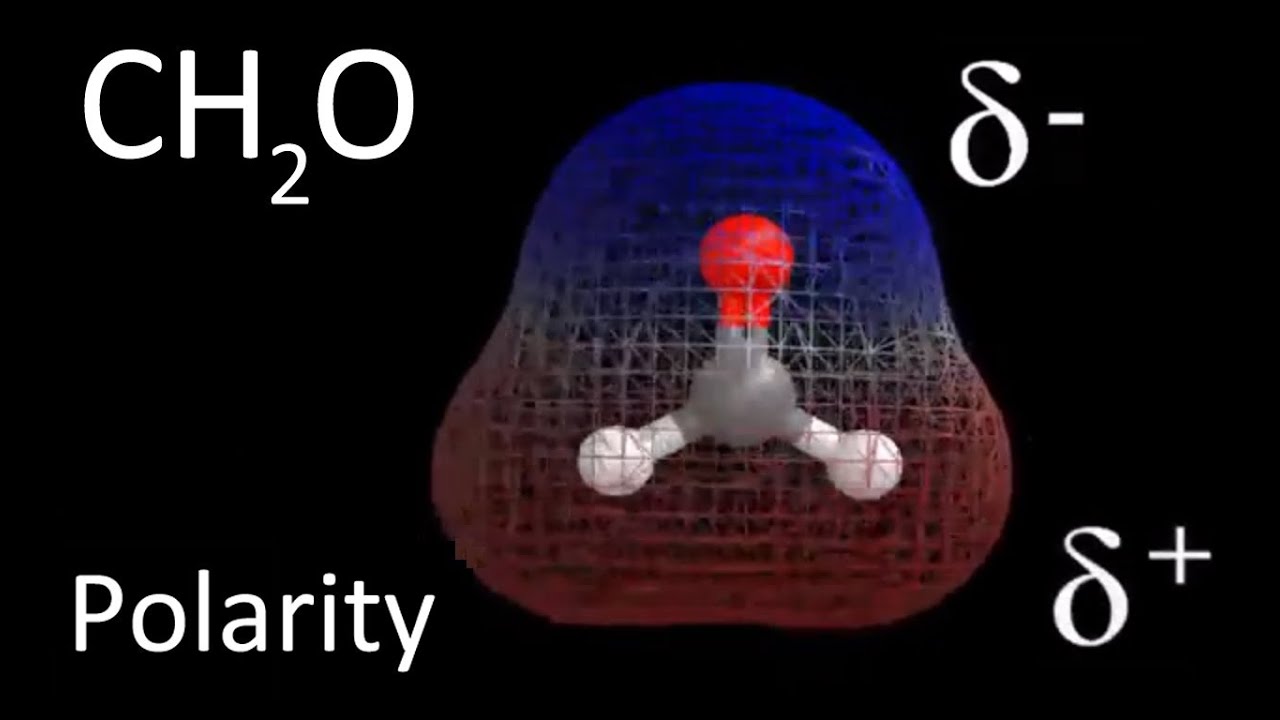
(CH2O) is a polar molecule. Because the oxygen atom is very electronegative, it is not non-polar (3.44). The hydrogen and carbon atoms can lose their partial positive charge and gain their partial negative charge from the oxygen atom. The molecule is polar because of this charge imbalance. Methanal has the chemical formula CH2O.
Is CH2O Polar or Nonpolar?
A molecule has polarity or is not determined by the difference in electronegativity and electron sharing** between two atoms in a covalent connection.
A molecule is said to as polar or nonpolar depending on how evenly two atoms share their electrons. Polar molecules are those in which this sharing is unequal.
The shared electron cloud from the entire molecule is strongly drawn to oxygen. As oppositely charged poles form, the molecule’s total charge distribution becomes unbalanced. With a net dipole moment value of 2.33 D, CH2O is polar.
| Molecule’s Name | Formaldehyde(CH2O) |
|---|---|
| Bond | Polar |
| Polar or Non Polar | Polar |
| Dipole Moment | 2.33 D |
| Bond Angle | 120º |
CH2O’s Electronegativity
(1 carbon), (1 hydrogen) and (2 oxygen atoms) make up the CH2O molecule. The electronegativity of carbon is 2.55, that of hydrogen is 2.2, and that of oxygen is 3.44. As a result, oxygen is the part of the formaldehyde molecule that is most electronegative.
Oxygen attracts the bound electron pair to one side of the atom. The oxygen atoms’ electron density rises due to the double bond that joins it to the carbon atom.
How Can I Check A Molecule’s Polarity?
Following are some elements that can assist in determining whether a molecule is polar or nonpolar:
The Dipole Moment:
It measures the separation between two electrical charges opposing one another. The polarity of the molecule increases with increasing dipole moment value. Polar molecules always have a dipole moment that is “non-zero,” whereas all nonpolar molecules have a dipole moment of “0.”
Electronegativity:
An atom’s ability to draw bound electron pairs to one side is referred to as electronegativity. The closer an atom attracts the bound electron pair, the higher its electronegativity.
Since oxygen has a high affinity for electrons, the shared electron cloud from the C=O bond is strongly drawn to it.
| Atom | Electronic Figures | Electrons |
|---|---|---|
| Carbon (6C) | 1s2 2s2 2p2 | 4 |
| Hydrogen (1H) | 1s1 | 1 |
| Oxygen (8O) | 1s2 2s2 2p4 | 6 |
Due to this significant difference in electronegativity, oxygen attracts the shared electrons from the two C-H bonds and the C=O electrons. Due to this overabundance of electrons, O acquires a partial negative charge (O-), whereas each of the C and H atoms acquires a partial positive charge (C+ and H++) due to their modest electron shortages.
Geometrical Shape:
Because polar molecules are asymmetric, the charges on their atoms appear to be dispersed unevenly.
As a result of their symmetrical form, nonpolar molecules maintain an identical charge balance across all of their atoms.
CH2O is an AB3-type molecule, according to the Valence Shell Electron Pair Repulsion (VSEPR) theory of chemical bonding. Three bond pairs (B) surround the core carbon atom (A), with no lone pair present.
Summary
Group 14 on the Periodic Table, or group IV A, is where carbon is located. The valence electron count is 4. The CH2O molecule forms two single covalent bonds with one hydrogen atom on either side and a double covalent link with an oxygen atom in the middle, using all of its valence electrons for chemical bonding.
FORMADEHYDE’S PHYSICAL CONDITIONS (CH2O)
- At normal temperature, formaldehyde appears as a colorless gas.
- It smells strongly oppressive and unpleasant.
- The weight of the molecules is 30.03 g/mol.
- Gaseous formaldehyde is very flammable.
- It has a boiling temperature of -19C and a melting point of -92C.
- It has a density of 0.8153 g/cm3 at -20 C, making it denser than water.
- In both water and acetone, CH2O is soluble. The solubility is 400 g/L in water.
- It has a vapor pressure above 1.
- Methanol has a dipole moment of 2.330 D.
- It is extremely poisonous, and serious skin damage is possible.
- Its aqueous solution evaporates, leaving a crystalline solid (paraformaldehyde).
- Unlike formaldehyde in gas form, which is not corrosive to carbon steel, formaldehyde in aqueous form is.
chemical characteristics of CH2O.
Chemical characteristics of water.
The following significant reaction occurs when methanol or formaldehyde are used:
-
oxidation process
-
low reaction.
-
reaction of self-oxidation and reduction.
-
Tollen’s reagent reaction.
-
response to Fehling’s remedy.
-
polymerization.
Summary
Methanal has the chemical formula CH2O. Formaldehyde is the name given to methane in commerce. It is a chemical that is colorless, pungent, and rapidly ignites. Perhaps the most basic compound in the family of organic chemistry is formaldehyde. Therefore, we must understand its chemical characteristics, such as its polarity.
formaldehyde uses
- Products made from formaldehyde include polyoxymethylene plastics, bakelite formaldehyde resins, urea-formaldehyde resins, 1,4-butanediol, melamine resins, and phenol-formaldehyde resins.
- The textile industry uses formaldehyde-based resins as a finisher to make fabric crease-resistant.
- To preserve biological specimens, formaldehyde and alcohol aqueous solutions are utilized.
- It combines with HNO to produce cyclonite, a highly explosive substance also utilized in World War II.
- Electrical system parts, car door panels, axles, and brake shoes are all made with methanol.
- Because it destroys most ■■■■■■ and bacteria, its aqueous solution is employed as a disinfectant.
- In cosmetics, formaldehyde releasers (also known as formaldehyde donors) are employed as biocides.
- Chemicals for paint are prepared with CH2O.
FREQUENTLY ASKED QUESTION (FAQs)
Q 1. Why is formaldehyde used as a preservative?
Formaldehyde is used as a preservative because it combines carbon, hydrogen, and oxygen; all these have antibacterial properties.
Q 2. How to neutralize formic acid?
Baking soda (NaHCO3) can neutralize acids; formic acid can also be neutralized by a thick paste of sodium bicarbonate water (baking soda).
Q 3. Is formaldehyde acidic?
No, it is not acidic, as its C-H bond is fairly stable, so it does not give H+ ions in the aqueous solution. Another reason is that it is not electron deficient and does not accept electrons. So many of the definitions of acid (Arrhenius, bronsted lowry, and lewis) do not apply to formaldehyde.
Q 4 Does the dipole moment of CH2O exist?
Some molecules with polar bonds have a net dipole moment due to their various three-dimensional configurations (HCl, CH2O, NH3, and CHCl3), whereas others do not because the bond dipole moments cancel (BCl3, CCl4, PF5, and SF6).
Q 5 C2H6 is it nonpolar or polar?
Because of its symmetrical form and absence of a dipole moment, it is a nonpolar molecule. Because it is nonpolar, ethane does not dissolve in water.
Q 6 Ethane is nonpolar, so why?
Due to the equal distribution of electrons within the molecule, ethane molecules are nonpolar covalent compounds. This molecule is nonpolar and evenly charged.
Q 7 How can I tell whether a bond is polar?
Although there are no hard and fast standards, the general rule is that a bond is termed nonpolar if the difference in electronegativities is less than or equal to 0.4 and polar if the difference is greater.
Q 8 What sort of bond has a nonpolar covalent?
Since they share electrons evenly, the bond between two hydrogen atoms is an example of a nonpolar covalent bond. The bond between two chlorine atoms illustrates a nonpolar covalent bond since they also share electrons equally.
Q 9 Which bond is most polar?
Since each molecule in the set contains hydrogen and another element, the bond that fluorine makes with the hydrogen molecule will be the most polar of the group since fluorine is the most electronegative element in the periodic table.
Q 10 Water is it nonpolar or polar?
A polar covalent molecule is a water.
Q 11 Why is CO2 nonpolar and H2O polar?
The polar bonds in CO2 and H2O are both two. The CO2 molecule is nonpolar because the dipoles in the linear form of the molecule cancel each other out. H2O is polar because of the polar links that cause a net dipole moment in the bent H2O molecule.
Q 12 Why is SO2 polar and CO2 nonpolar?
Since the various dipole moments of the $CO$ bond cancel each other out and $C O 2$ is a linear molecule, the resultant dipole moment is zero. In contrast, because $S O 2$ is an angular molecule, it has a net dipole moment. In light of this, $SO$ is polar while $CO$ is nonpolar.
Q 13 Which CO2 SO2 SO3 is nonpolar?
While SO2 is a polar molecule, CO2 is not.
Q 14 Ethane is nonpolar, so why?
Due to the equal distribution of electrons within the molecule, ethane molecules are nonpolar covalent compounds. This molecule is nonpolar and evenly charged.
Q 15 How can I tell whether a bond is polar?
Although there are no hard and fast standards, the general rule is that a bond is termed nonpolar if the difference in electronegativities is less than or equal to 0.4 and polar if the difference is greater.
Conclusion
CH2O is Polar; the reason for its polarity is the highly electronegative oxygen atom and the uneven sharing of electrons. Its dipole moment is non-zero (2.330 D), which is also the reason for its polarity. The structure of formaldehyde is asymmetrical (trigonal planar), having a bond angle of 120 degrees.