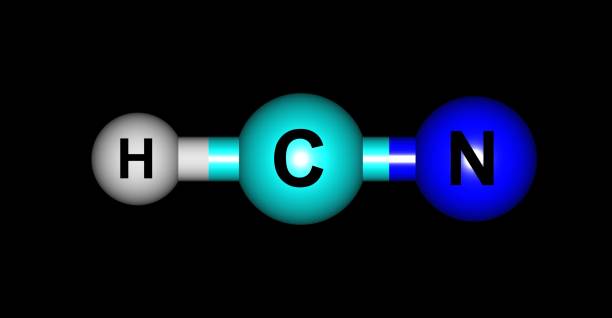
HCN Acid Name is Formonitrile (HCN), also commonly known as hydrogen cyanide, is a very volatile, colorless, and highly toxic liquid. Hydrocyanic acid, also known as prussic acid, is a solution of hydrogen cyanide in water used to make dyes.
 HCN Acid Name
HCN Acid Name
One of the most toxic liquids known to man is Hydrogen Cyanide (HCN), which has a boiling point of 26°C (79°F) and a freezing point of -14C (-7F). Hydrocyanic acid, often known as prussic acid, is a water-based solution of hydrogen cyanide. Hydrogen Cyanide is very volatile, colorless, and highly deadly.
Carl Wilhelm Scheele, a Swedish scientist, developed it in 1782 by preparing it from the Prussian blue pigment. It is common practice to employ cyanide-containing chemicals in a wide variety of chemical processes, including Fumigation, case hardening, electroplating, and the concentration of ores, to name a few.
Acrylonitrile, the raw material for acrylic fibers, synthetic rubber, and plastics, is also made. Hydrogen cyanide gas can provide a deadly dose of capital punishment. An adult human can tolerate hydrogen cyanide concentrations of 50–60 parts per million for an hour, but exposure to 200–500 elements per million air for 30 minutes is usually lethal.
Hazardous hydrogen cyanide hinders cellular oxidative reactions, making it highly toxic. It is possible to obtain trace amounts of hydrogen cyanide from plants with carbohydrates. Laboratory and industrial-scale production of hydrogen cyanide rely on one of three primary methods:
-
Using sulfuric acid to treat sodium cyanide
-
A combination of methane and ammonia is oxidized using a catalyst.
-
Breaking down into its parts (HCONH2)
 Formation Of Aldehydes And Ketones
Formation Of Aldehydes And Ketones
It’s accessible to polymerize hydrogen cyanide in the presence of essential compounds like ammonia or sodium cyanide, but in its pure state, it’s pretty inert. Toxicities prevent being widely employed as a solvent even though it is an excellent salt solvent.
Ore extraction, electrolysis, and steel treatment all use these salts. Cyanohydrins formed from aldehydes and ketones are intermediates in various organic synthesis processes. In many cases, ethylene oxide is used to produce an intermediate product transformed to acrylonitrile (CH2=CHCN).
An aqueous solution containing 2–10 percent hydrogen cyanide is the most common commercial form. Hydrocyanic acid has a chemical formula of HCN. Carbon dioxide is lighter than air and evaporates more quickly when mixed with hydrocyanic acid.
 HCN’s Various Applications
HCN’s Various Applications
In warehouses, grain storage troughs, greenhouses, and holds, it is used to kill pests like rodents of ships where its high toxicity and ability to enter hidden areas are helpful.
-
Cyanide salts, acrylonitrile, and dyes are all products of this process. As a horticultural fumigant, it can also be applied to crops.
-
There are various compounds where this is an essential precursor.
-
The sp hybridization of the carbon atom occurs in the HCN structure.
-
C-H and C-N are the only sigma bonds that exist. The number of hybrid orbitals produced is proportional to the number of sigma bonds present.
-
Hydrocyanic acid is converted to sodium cyanide and water by reacting with a sodium hydroxide base. Below is the chemical equation.
-
When HCN and NaOH are combined, NaCN and water result.
-
Sodium hydroxide interacts with hydrogen cyanide to produce potassium cyanide and water. Below is the chemical equation.
-
In this case, HCN and KOH form H2O and KCN.
-
To use hydrocyanic acid as a suicide or murder weapon is to employ one of the fastest-acting poisons on the market.
-
Hydrocyanic acid is breathed, causing dizziness, a feeling of suffocation, nausea, and other unpleasant symptoms.
The production of cyanide salts, acrylonitrile, and colors relies on hydrogen cyanide. It can be used as a horticultural fumigant on crops. These products are used in mining, synthesis of chemicals, synthetic textiles, dyes, and insecticides.
Summary
Hydrogen cyanide aqueous solutions break down to generate ammonium formate over time. It’s a highly toxic, colorless gas with an almond-like scent. Keeping and transporting it are strictly forbidden because it is a highly toxic, transparent liquid.
 Systemic Chemical Asphyxiant
Systemic Chemical Asphyxiant
Chemical asphyxiant hydrogen cyanide (AC) is systemic. Nearly all of the body’s organs have trouble using oxygen generally because of it. Hydrogen cyanide (AC) poisoning can be lethal in minutes. All body systems are affected, especially those most vulnerable to oxygen deprivation.
The brain, heart, blood arteries, and lungs comprise the central nervous system (lungs). The chemical warfare agent hydrogen cyanide (AC) (military designation, AC).
 Commercial Use
Commercial Use
Fungicides and pesticides are made commercially for Fumigation, electroplating and mining, synthesis of chemicals, synthetic fibers, and dyes and insecticides. It has a bitter, burning flavor and is frequently mixed with liquids like vinegar or rubbing alcohol.
Many people" cannot smell the "bitter almond odor of hydrogen cyanide (AC) gas, which others describe as having a musty “old sneakers scent.” Because of this, the odor does not provide a significant warning “f hazardous amount.”
 Is HCN An Acid Or A Base?
Is HCN An Acid Or A Base?
Contact with hydrogen cyanide, one of chemistry’s most lethal chemicals, results in death almost immediately due to the compound’s ability to prevent oxygen from reaching vital tissues. The chemical formula for this substance is HCN. This colorless gas has a strong, pungent odor that can irritate the eyes and lungs, in addition to causing inflammation.
Acidity: HCN has a pKa of 9.5. Hydrocyanic acid is a solution that contains one hydrogen ion and one cyanide anion (CN–). When hydrogen cyanide is dissolved in water, it releases one proton (H+) and one CN– ion into the solution.
 Why Is HCN An Acid And Not A Base?
Why Is HCN An Acid And Not A Base?
Many of the acidic compounds begin with hydrogens, such as hydrochloric acid (H2SO4), HCl, HBr, nitrLet’sid (HNO3), and carbon dioxide (H2CO3). Most basic chemicals have hydroxide groups (OH) such as NaOH, KOH, LiOH, etc.
Hydrogen and OH–ions are formed when acidic and basic substances are dissolved in water. By and large, the current thinking on the acid-base relationship holds that acids provide H+, whereas bases provide an OH– ion. H+ and CN– ions are produced in the solution when HCN is dissolved in water.
The acidic character of HCN is due to the presence of an H+ ion in an aqueous solution. The two most critical acid-base hypotheses can now be used to determine if HCN is an acid or a base.
-
Theory of Arrhenius
-
Theory of Bronsted and Lowry
 Arrhenius theory
Arrhenius theory
A chemical behaves like acid because it releases a hydrogen ion. When a substance dissolves in water, it releases an OH– ion, indicating a base. It is also possible for a chemical to be acidic or basic, depending on whether or not it raises or lowers the concentration of H+ in the solution.
Verifying Arrhenius acid theory is as simple as counting hydrogen ions in solution before and after the reaction. Adding hydrogen cyanide to an aqueous solution releases a proton, which increases the final solution’s hydrogen ion concentration. HCN reacts with the two hydrogens on the left side, transforming them into three. Hydrogen ion concentrations have increased, without a doubt.
 Bronsted-Lowry Theory
Bronsted-Lowry Theory
Bases are those substances that absorb the provided proton and then make conjugate acids by adding one more proton to themselves. Acidic materials lend protons to other species and lose one proton to generate conjugate bases. This is what Let’seant by the term “base.”
Let’s take a look at the reaction between HCN and H2O. By giving up one of its protons, HCN becomes a conjugate base (CN–), which then becomes a conjugate acid (H3O+) by giving up one of its protons.
 Weak Acidity Of HCN
Weak Acidity Of HCN
When dissolved in water, H doesn’t entirely ionize to produce H+ ions, making it a weak acid, which means that when HCN dissolves in water, some of the ions remain in the solution. The final solution contains fewer hydrogen ions since just a portion doesn’t dissociate.
The question is why HCN partially dissolves in the solution and acquires the weak acid strength. It is necessary to consider several aspects that influence acidic compound strength to grasp this fully. Only two elements are considered that could affect the compound’s structure.
-
Electronegativity
We took notes to grasp better how these two factors work together to establish the compound’s strength.
-
The more substantial the electronegativity gap between the atoms, the more polar the connection becomes.
-
Deprotonation from polar molecules is more straightforward in water solution than deprotonation from non-polar molecules.
-
Acidic strength increases with molecule polarity because the proton can quickly exit the molecule.
-
Acidity increases when hydrogen is bound to an electronegative atom.
Carbon has an electronegativity of 2.55, hydrogen has a value of 2.2, and nitrogen has a value of 3.04. A single link connects hydrogen to carbon, while three bonds connect carbon to nitrogen in an HCN molecule.
Summary
Any substance that alters the concentration of hydrogen ions (H+) in the solution can be acidic or basic. Protons released when hydrogen cyanide is dissolved in an aqueous solution enhance the concentration of hydrogen ions in the final solution.
 Cyanide Poisoning
Cyanide Poisoning
Cyanide Ingestion of cyanide salts or inhalation of hydrogen cyanide can result in poisoning. Hydrocyanic acid (HCN) makes acrylic fibers, synthetic rubber, and plastic. Fumigation, case hardening of iron and steel, electroplating, and mineral concentration are just a few of the chemical processes that make use of cyanides.
Cyanide-producing chemicals can be found in wild cherry pits and other seeds. Hazardous hydrogen cyanide hinders the cells’ oxidative activities, making it highly toxic. Ingestion of 300 milligrams of salts or inhalation of 100 milligrams of hydrogen cyanide can quickly result in death.
The symptoms of acute Cyanide poisoning include nausea, vomiting, dizziness, and loss of consciousness. Toxic levels of 200–500 parts per 1,000,000 air units for 30 minutes are also frequently lethal. The human body quickly breaks down Cyanide into harmless sulfonamides after sublethal exposure.
Due to the poison’s rapid action, recovery from poisoning is contingent on short antidotes. Treatments such as amyl nitrite, sodium nitrite, and 25 percent sodium thiosulfate solution can help prevent fatalities.
 Cyanide Poisoning Sources
Cyanide Poisoning Sources
Despite its rarity, Cyanide is a potent toxin. It operates by preventing the body from absorbing its oxygen to sustain itself. Hydrogen cyanide gas, crystalline solids, potassium cyanide, and sodium cyanide are some of the cyanide compounds that can be toxic.
-
A variety of different things can cause Cyanide poisoning.
-
Firefighters’ smoke inhalation
-
Cyanide-using industries (photography, chemical research, synthetic plastics, metal processing, and electroplating),
-
The term “plants” refers to (such as apricot pits and a type of potato called cassava),
-
Chemotherapy drug laetrile
 Symptoms And Signs
Symptoms And Signs
If you suspect cyanide poisoning, you should look for these signs and symptoms:
| Broad-Based Mediocrity | |
| Confusion | |
| Irrational Conduct | |
| Drowsiness | |
| Coma | |
| Feeling Of Exhaustion | |
| Headache | |
| Dizziness | |
| Vomiting | |
| Pain in Abdomen | |
| Seizures |
 Antimony Poisoning
Antimony Poisoning
Certain antimony compounds can cause antimony poisoning and have detrimental effects on the human body’s tissues. Chemical poisoning is similar to this. Antimony poisoning is connected to acidic fruit juices containing antimony oxide dissolved from poor enamelware glaze.
Anti-helminthic and anti-fungal medicines, such as tartar emetic, can produce antimony poisoning (antimony and potassium tartrate). Antimony and chemical are toxic when coupled with enzymes (the organic catalysts of the cell). There is no evidence that antimony is hazardous at work.
 Fluorosis
Fluorosis
Chronic fluorine poisoning changes the skeleton and hardens tendons and ligaments. This is fluorosis. Optimal fluoride exposure promotes tooth and bone growth; excessive fluoride consumption causes death. Mild chronic exposure causes mottling in children’s teeth but not in their bones.
Fluorine gradually replaces calcium in the bones, making them fragile and crumbly and turning them chalky white. New bone grows in places where it shouldn’t. Nerve compression in the spinal cord can cause neurological symptoms such as stiffness and inability to move the spine.
Insecticide, aluminum-mining, and phosphate-fertilizer employees and those who reside in locations with naturally high fluoride water supplies are among the groups at risk of long-term exposure from pollution in the air. This type of exposure does not cause fluorosis in the Western Hemisphere, but it is prevalent in portions of India and Arabia.
Summary
It’s a potent toxin, even though Cyanide is quite rare. By stopping the body from taking in oxygen, it functions. Cyanide substances that can be harmful include hydrogen cyanide gas, crystalline solids, potassium cyanide, and sodium cyanide.
 Frequently Asked Question - FAQs
Frequently Asked Question - FAQs
The following are some of the most frequent inquiries we receive regarding Hydrogen Cyanide (HCN)
 What is the scientific name for hydrocyanic acid?
What is the scientific name for hydrocyanic acid?
The name of the acid is the root of the anion, followed by the suffix -ic if the anion ends in -ate. Because Cl is the chloride ion, HCl is hydrochloric acid. It is hydrocyanic acid because CN is the cyanide ion in HCN.
 Cyanide is what type of acid?
Cyanide is what type of acid?
Industrial-scale hydrocyanic acid (HCN), commonly known as hydrogen cyanide or HCN, results in a highly volatile liquid. Acidification of cyanide salts yields it. The organic cyanides known as nitriles have a cyanide moiety.
 What is the purpose of hydrocyanic acid?
What is the purpose of hydrocyanic acid?
Fumigation, iron, steel case hardening, electroplating, and ore concentration are just a few chemical processes that utilize hydrogen cyanide and its derivatives. As well as producing acrylonitrile, which is used to make synthetic rubber and plastics, it is also used to create acrylonitrile itself.
 Is HCN a powerful electrolyte or a weak one?
Is HCN a powerful electrolyte or a weak one?
Only a tiny percentage of HCN molecules ionize when dissolved in water. The fact that HCN is an acid and most pupils have memorized the most frequent strong acids tells us this (vital electrolytes).
 What is HCN’s electronegativity?
What is HCN’s electronegativity?
Because of the difference in electronegativity between the two atoms, the C-N bond is somewhat polar covalent connection. While the electronegativity of the N is 3.0, that of the C is only 2.5. There is a difference of 0.5, which would result in a somewhat polar covalent bond.
 Is H2CO3 a weak acid?
Is H2CO3 a weak acid?
When carbon dioxide dissolves in water, it forms carbonic acid. Carbonic acid is H2CO3. A carboxyl group links two hydroxyl groups at the molecule’s core. Dissociation or partial ionization are all terms that describe what happens in a solution to a weak acid.
 What is the acidity of urine?
What is the acidity of urine?
The results of these tests can be compared to the examples above. The usual pH range is between 4.6 and 8.0. The content of average values may be slightly varied in different laboratory settings. Some labs use a variety of metrics, while others focus on a single sample.
 Is the molecule HCN polar?
Is the molecule HCN polar?
As a result of the enormous disparity in electronegativity between N and H, hydrogen cyanide (HCN) is a polar molecular compound. This structure comprises two oppositely-polar bonds whose polarities line up.
 What is the liquid that contains Urobilinogen?
What is the liquid that contains Urobilinogen?
Your liver produces bilirubin, a yellowish chemical that aids in the breakdown of red blood cells. Most people’s urine contains some of this Urobilinogen. The amount of Urobilinogen in a urine sample can be detected by decreasing bilirubin, which leads to the creation of Urobilinogen.
 How does HCN electronegativity work?
How does HCN electronegativity work?
Because of the difference in electronegativity between the two atoms, the C-N bond is a somewhat polar covalent connection. While the electronegativity of the N is 3.0, the C is only 2.5. There is a difference of 0.5, which would result in a somewhat polar covalent bond.
Conclusion
The chemical asphyxiant hydrogen cyanide (AC) has a widespread distribution. As a result, nearly all of the body’s organs have difficulty utilizing oxygen in a generalized manner. Poisoning with hydrogen cyanide (AC) can be fatal within minutes of exposure.
The chemical warfare agent hydrogen cyanide (AC) is a cyanide compound (military designation, AC).> It affects all body systems, especially those most vulnerable to oxygen deprivation. The central nervous system comprises the brain, the heart, the blood vessels, and the lungs (lungs).