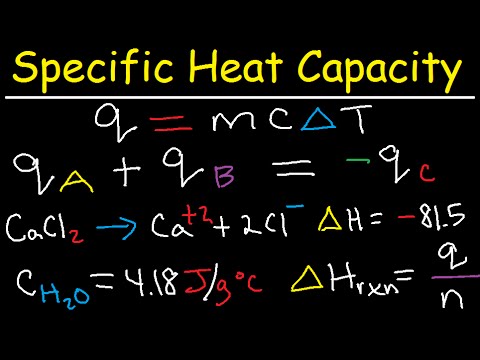
Specific heat equation= Heat energy = (mass of the item or substance) × (specific heat) × (Change in temperature)
Q = m × c × ΔT
Or on the other hand
Q = mcΔT
Q is how much provided or deducted heat (in joules), m is the mass of the example, and ΔT is the contrast between the underlying and last temperatures. Heat capacity is estimated in J/(kg·K).
What Is Specific Heat?
This specific heat adding machine is an apparatus that decides the heat capacity of a heated or a cooled test.
Specific heat is how much thermal energy you really want to supply to an example gauging 1 kg to build its temperature by 1 K. Peruse on to figure out how to apply the heat capacity formula accurately to get a substantial outcome.
The heat capacity estimates how much heat important to raise the temperature of an item or framework by one degree Celsius.
Specific heat is how much thermal energy you really want to supply to an example gauging 1 kg to expand its temperature by 1 K.
Heat capacity (for the most part indicated by a capital C, frequently with addendums), or thermal capacity, is the quantifiable actual amount that describes how much heat expected to change a substance’s temperature by a given sum.
In SI units, heat capacity is communicated in units of joules per kelvin (J/K).
An item’s heat capacity is characterized as the proportion of how much heat energy moved to an item to the subsequent expansion in temperature of the article.
C=QΔT.C=QΔT.
Heat capacity is a broad property, so it scales with the size of the framework. An example containing double how much substance as another example requires the exchange of two times as much heat (Q) to accomplish a similar change in temperature (ΔT).
For instance, in the event that it takes 1,000 J to heat a square of iron, it would require 2,000 J to heat a second square of iron with double the mass as the first.
What is SI unit of specific heat capacity?:
Global Arrangement Of Unit
The SI unit for specific heat capacity is joule per kelvin per kilogram ((J/K)/kg, J/(kg⋅K), J⋅K−1⋅kg−1, and so forth) Since an augmentation of temperature of one degree Celsius is equivalent to an addition of one kelvin, that is equivalent to joule per degree Celsius per kilogram (J⋅kg−1⋅°C−1).
At times the gram is utilized rather than kilogram for the unit of mass: 1 J⋅g−1⋅K−1 = 0.001 J⋅kg−1⋅K−1.
The specific heat capacity of a substance (per unit of mass) has aspect L2⋅Θ−1⋅T−2, or (L/T)2/Θ. Hence, the SI unit J⋅kg−1⋅K−1 is comparable to meter squared each second squared per kelvin (m2⋅K−1⋅s−2)
The Measurement Of Heat Capacity
The heat capacity of most frameworks is anything but a constant. Rather, it relies upon the state factors of the thermodynamic framework under study.
Specifically, it is subject to temperature itself, as well as on the pressure and the volume of the framework, and the manners by which pressures and volumes have been permitted to change while the framework has passed starting with one temperature then onto the next.
The justification behind this is that pressure-volume work done to the framework raises its temperature by an instrument other than heating, while pressure-volume work done by the framework ingests heat without raising the framework’s temperature.
(The temperature reliance is the reason the definition a calorie is officially the energy expected to heat 1 g of water from 14.5 to 15.5 °C rather than for the most part by 1 °C. )
Various measurements of heat capacity can hence be performed, most usually at constant pressure and constant volume. The qualities accordingly estimated are normally subscripted (by p and V, individually) to show the definition. Gases and fluids are regularly likewise estimated at constant volume.
Measurements under constant tension produce bigger qualities than those at constant volume on the grounds that the constant pressure esteems likewise incorporate heat energy that is utilized to take care of business to grow the substance against the constant pressure as its temperature increments.
This distinction is especially striking in gases where values under constant tension are commonly 30% to 66.7% more noteworthy than those at constant volume.
Induction Of Specific Heat Formula
Q = alludes to the heat energy in Joules (J)
m = alludes to the mass of the substance in kilogram (kg)
c = alludes to the specific heat in joules per kilogram (J/kg⋅k)
Δ = alludes to the image of progress
Δt = alludes to the adjustment of temperature in kelvins (K)
Summary
Measurements under constant tension produce bigger qualities than those at constant volume on the grounds that the constant pressure esteems likewise incorporate heat energy that is utilized to take care of business to grow the substance against the constant pressure as its temperature increments.
Step By Step Instructions To Ascertain Specific Heat
Specific heat is how much energy expected to raise one gram of an unadulterated substance by one degree Centigrade. The specific heat of a substance is reliant upon the two its sub-atomic design and its stage.
The revelation of specific heat ignited the investigations of thermodynamics, the investigation of energy change including heat and crafted by a framework. Specific heat and thermodynamics are utilized broadly in science, atomic designing, and streamlined features.
As well as in daily existence in the radiator and cooling arrangement of a vehicle.
To know how to compute specific heat, simply follow these steps:
-
Decide if you need to heat up the example (give it some thermal energy) or cool it down (remove some thermal energy).
-
Embed how much energy provided as a positive worth. To chill off the example, embed the deducted energy as a negative worth. For instance, say that we need to diminish the example’s thermal energy by 63,000 J.
-
Then, at that point, Q = - 63,000 J.
-
Choose the temperature contrast between the underlying and last condition of the example and type it into the heat capacity number cruncher. Assuming the example is chilled off, the distinction will be negative, and whenever heated up - positive.
-
Suppose we need to chill the example off by 3 degrees.
Then, at that point, ΔT = - 3 K. You can likewise go to cutting edge mode to type the underlying and last upsides of temperature physically.
-
Decide the mass of the example. We will expect to be m = 5 kg.
-
Compute specific heat as c = Q/(mΔT). In our model, it will be equivalent to c = - 63,000 J/(5 kg * - 3 K) = 4,200 J/(kg·K). This is the average heat capacity of water.
Heat Capacity Formula
The formula for specific heat resembles this:
c = Q/(mΔT)
Q is how much provided or deducted heat (in joules), m is the mass of the example, and ΔT is the distinction between the underlying and last temperatures. Heat capacity is estimated in J/(kg·K).
The ordinary upsides of specific heat equation, given below:
Values Of Specific Heat
You don’t have to involve the heat capacity mini-computer for most normal substances. The upsides of specific heat for probably the most well known ones are recorded below:
| Typical values | |
|---|---|
| Ice: | 2,100 J/(kg·K) |
| Water: | 4,200 J/(kg·K) |
| Water fume: | 2,000 J/(kg·K) |
| Basalt: | 840 J/(kg·K) |
| Rock: | 790 J/(kg·K) |
| Aluminum: | 890 J/(kg·K) |
| Iron: | 450 J/(kg·K) |
| Copper: | 380 J/(kg·K) |
| Lead: | 130 J/(kg·K) |
Having this data, you can likewise compute how much energy you want to supply to an example to increment or lessening its temperature. For example, you can check how much heat you really want to carry a pot of water to the bubble to cook some pasta.
Thinking about what the outcome really implies? Attempt our potential energy adding machine to check how high you would raise the example with this measure of energy. Or on the other hand check how quick would the example be able to move with this motor energy number cruncher.
How To work Out Specific Heat Capacity?
Observe the underlying and last temperature as well as the mass of the example and energy provided.
-
Take away the last and beginning temperature to get the adjustment of temperature (ΔT).
-
Duplicate the adjustment of temperature with the mass of the example.
-
Partition the heat provided/energy with the item.
The formula is C = Q/(ΔT ⨉ m).
Thermodynamic Relations And Definition Of Heat Capacity
The inward energy of a shut framework changes either by adding heat to the framework or by the framework performing work. Reviewing the main law of thermodynamics,
dU=δQ−δWdU=δQ−δW.
For function because of an increment of the framework volume we might compose,
dU=δQ−PdVdU=δQ−PdV.
On the off chance that the heat is added at constant volume, the second term of this connection evaporates and one promptly acquires
(∂U∂T)V=(∂Q∂T)V=CV(∂U∂T)V=(∂Q∂T)V=CV.
This characterizes the heat capacity at constant volume, CV. One more valuable amount is the heat capacity at constant pressure, CP. With the enthalpy of the framework given by
H=U+PVH=U+PV,
Our equation for dU changes to
dH=δQ+VdPdH=δQ+VdP,
furthermore hence, at constant pressure, we have
(∂H∂T)P=(∂Q∂T)P=CP(∂H∂T)P=(∂Q∂T)P=CP.
Specific Heat
The specific heat is an escalated property that depicts how much heat should be added to a specific substance to raise its temperature.
Specific Heat For An Ideal Gas At Constant Pressure And Volume
The heat capacity at constant volume of nR = 1 J·K−1 of any gas, including an ideal gas is:
(∂U∂T)V=cv(∂U∂T)V=cv
This addresses the dimensionless heat capacity at constant volume; it is for the most part a component of temperature because of intermolecular powers. For moderate temperatures, the constant for a monoatomic gas is cv=3/2 while for a diatomic gas it is cv=5/2 (see ). Naturally visible measurements on heat capacity give data on the tiny design of the atoms.
The heat capacity at constant pressure of 1 J·K−1 ideal gas is:
(∂H∂T)V=cp=cv+R(∂H∂T)V=cp=cv+R
where H=U+pV is the enthalpy of the gas.
Estimating the heat capacity at constant volume can be restrictively challenging for fluids and solids.
That is, little temperature changes normally require enormous pressures to keep a fluid or strong at constant volume (this infers the containing vessel should be almost unbending or possibly exceptionally solid).
It is simpler to quantify the heat capacity at constant pressure (permitting the material to grow or contract uninhibitedly) and settle for the heat capacity at constant volume utilizing numerical connections got from the essential thermodynamic regulations.
Using the Fundamental Thermodynamic Relation we can show:
Cp−CV=T(∂P∂T)V,N(∂V∂T)p,NCp−CV=T(∂P∂T)V,N(∂V∂T)p,N
where the fractional subordinates are taken at: constant volume and constant number of particles, and at constant pressure and constant number of particles, individually.
The heat capacity proportion or adiabatic list is the proportion of the heat capacity at constant pressure to heat capacity at constant volume. It is in some cases otherwise called the isentropic extension factor:
γ=CPCV=cpcvγ=CPCV=cpcv
For an optimal gas, assessing the halfway subordinates above as per the equation of state, where R is the gas constant for an ideal gas yields:
pV=RTpV=RT
Cp−CV=T(∂P∂T)V(∂V∂T)p_Cp−CV=T(∂P∂T)V(∂V∂T)p
Cp−CV=−T(∂P∂V)V(∂V∂T)2■■■−CV=−T(∂P∂V)V(∂V∂T)p2
P=RTVn→(∂P∂V)T=−RTV2=−PVP=RTVn→(∂P∂V)T=−RTV2=−PV
V=RTPn→(∂V∂T)2p=R2P2V=RTPn→(∂V∂T)p2=R2P2
subbing:
−T(∂P∂V)V(∂V∂T)2p=−T−PVR2P2=R−T(∂P∂V)V(∂V∂T)p2=−T−PVR2P2=R
This equation lessens just to what exactly is known as Mayer’s connection:
CP−CV=RCP−CV=R.
It is a basic equation relating the heat limits under constant temperature and under constant tension.
Tackling Problems With Calorimetry
Calorimetry is utilized to quantify how much heat created or consumed in a synthetic response.
Heat Transfer, Specific Heat, And Heat Capacity
We learned in the past area that temperature is relative to the normal dynamic energy of iotas and particles in a substance, and that the normal interior active energy of a substance is higher when the substance’s temperature is higher.
Assuming two items at various temperatures are gotten contact with one another, energy is moved from the more sweltering article (that is, the item with the more prominent temperature) to the colder (lower temperature) object, until the two articles are at a similar temperature.
There is no net heat move once the temperatures are equivalent in light of the fact that how much heat moved from one item to the next is equivalent to how much heat returned. One of the significant impacts of heat move is temperature change: Heating expands the temperature while cooling diminishes it.
Tests show that the heat moved to or from a substance relies upon three considers the change the substance’s temperature, the mass of the substance, and certain actual properties connected with the period of the substance.
The equation for heat move Q is
Q = mcΔT,Q = mcΔT,
where m is the mass of the substance and ΔT is the adjustment of its temperature, in units of Celsius or Kelvin. The image c represents specific heat, and relies upon the material and stage.
The specific heat is how much heat important to change the temperature of 1.00 kg of mass by 1.00 ºC. The specific heat c is a property of the substance; its SI unit is J/(kg ⋅⋅ K) or J/(kg ⋅⋅ °C°C ).
The temperature change ( ΔTΔT ) is something similar in units of kelvins and degrees Celsius (however not degrees Fahrenheit).
Specific heat is firmly connected with the idea of heat capacity. Heat capacity is how much heat important to change the temperature of a substance by 1.00 °C°C .
In equation structure, heat capacity C is C=mcC=mc, where m is mass and c is specific heat. Note that heat capacity is equivalent to specific heat, however with next to no reliance on mass.
Thus, two items comprised of a similar material yet with various masses will have different heat limits. This is on the grounds that the heat capacity is a property of an item, however specific heat is a property of any article made of a similar material.
Heat Capacity Of Water
Heat capacity, proportion of heat consumed by a material to the temperature change. It is normally communicated as calories per degree as far as the real measure of material being thought of, most ordinarily a mole (the atomic load in grams).
The heat capacity in calories per gram is called specific heat.
The meaning of the calorie depends on the specific heat of water, characterized as one calorie for every degree Celsius.
At adequately high temperatures, the heat capacity per molecule will in general be no different for all components. For metals of higher nuclear weight, this estimation is as of now a decent one at room temperature, leading to the law of Dulong and Petit.
For different materials, heat capacity and its temperature variety rely upon contrasts in energy levels for particles (accessible quantum states).
Heat limits are estimated with some assortment of calorimeter, and, utilizing the formulation of the third law of thermodynamics, heat-capacity measurements became significant for of deciding the entropies of different materials.
Summary
This specific heat adding machine is an apparatus that decides the heat capacity of a heated or a cooled test. Specific heat is how much thermal energy you want to supply to an example gauging 1 kg to expand its temperature by 1 K. Peruse on to figure out how to apply the heat capacity formula accurately to acquire a legitimate outcome.
Frequently Asked Questions FAQ’s
Some of the questions asked by reader about specific heat equation are given below:
1. What is specific heat capacity at constant volume?
The specific heat capacity is the heat or energy expected to transform one unit mass of a substance of a constant volume by 1 °C. The formula is Cv = Q/(ΔT ⨉ m).
2. What is the formula for specific heat?
The formula for specific heat capacity, C, of a substance with mass m, is C = Q/(m ⨉ ΔT). Where Q is the energy added and ΔT is the adjustment of temperature. The specific heat capacity during various cycles, like constant volume, Cv and constant pressure, Cp, are connected with one another by the specific heat proportion, ɣ= Cp/Cv, or the gas constant R = Cp - Cv.
3. What are the units for specific heat capacity?
Specific heat capacity is estimated in J/kg K or J/kg C, as it is the heat or energy expected during a constant volume interaction to change the temperature of a substance of unit mass by 1 °C or 1 °K.
4. What is the specific heat capacity worth of water?
The specific heat of water is 4179 J/kg K, how much heat expected to raise the temperature of 1 g of water by 1 Kelvin.
5. What are the royal units for specific heat?
Specific heat is estimated in BTU/lb °F in magnificent units and in J/kg K in SI units.
6. What is the specific heat capacity worth of copper?
The specific heat of copper is 385 J/kg K. You can utilize this worth to assess the energy expected to heat a 100 g of copper by 5 °C, i.e., Q = m x Cp x ΔT = 0.1 * 385 * 5 = 192.5 J.
7. What is the specific heat capacity worth of aluminum?
The specific heat of aluminum is 897 J/kg K. This worth is practically 2.3 seasons of the specific heat of copper. You can utilize this worth to assess the energy expected to heat a 500 g of aluminum by 5 °C, i.e., Q = m x Cp x ΔT = 0.5 * 897* 5 = 2242.5 J.
8. How would I ascertain specific heat?
Ascertain specific heat as c = Q/(mΔT) . In our model, it will be equivalent to c = - 63,000 J/(5 kg * - 3 K) = 4,200 J/(kg·K) .
9. What is the formula for specific inactive heat?
The specific idle heat (L) of a material, is a proportion of the heat energy (Q) per mass (m) delivered or consumed during a stage change. is characterized through the formula Q = mL.
10. What is interior energy equation?
Change in Internal Energy Formula
The Change in Internal Energy Formula is: ΔU = Q + W. Here, U = the complete change in inner energy inside the framework. Q = the heat traded between a framework and its environmental factors (outside the framework)
11. What is a heat energy?
Heat energy is the aftereffect of the development of small particles called iotas, atoms or particles in solids, fluids and gases. Heat energy can be moved starting with one article then onto the next. The exchange or stream because of the distinction in temperature between the two items is called heat.
12. What is a unit of heat energy called?
Calorie, a unit of energy or heat differently characterized. Another calorie, a unit of heat energy, is the International Table calorie (IT calorie), initially characterized as 1/860 global watt-hour. It is equivalent to 4.1868 joules and is utilized in designing steam tables.
13. How would you make heat energy?
Thermal energy (additionally called heat energy) is delivered when an ascent in temperature makes particles and atoms move quicker and crash into one another. The energy that comes from the temperature of the heated substance is called thermal energy.
14. What are 3 kinds of heat?
Heat can be moved in three ways: by conduction, by convection, and by radiation.
Conduction is the exchange of energy starting with one atom then onto the next by direct contact.
Convection is the development of heat by a liquid like water or air.
Radiation is the exchange of heat by electromagnetic waves.
Conclusion
Heat capacity equation — The equation for specific heat looks like this: c = Q / (mΔT). Q is the amount of supplied or subtracted heat (in joules).
Specific heat equation= Heat energy = (mass of the item or substance) × (specific heat) × (Change in temperature)
Q = m × c × ΔT
Or on the other hand
Q = mcΔT
Related Articles
https://howtodiscuss.com/t/science-formula/102201
https://howtodiscuss.com/t/theoretical-physics/141500
https://howtodiscuss.com/t/how-to-solve-system-of-equations/115809
https://howtodiscuss.com/t/electric-heating-system/164726
https://howtodiscuss.com/t/types-of-heat/110605