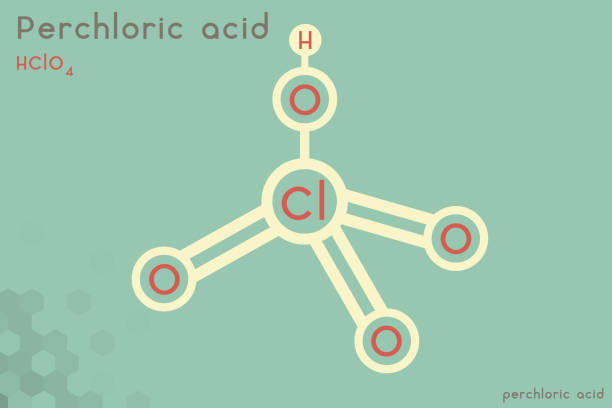
Perchloric Acid formula is HClO₄. Perchloric acid is an inorganic acid that is normally synthesized as an aqueous solution and then used to make perchlorate salts for use in the chemical industry.
 Perchloric acid:
Perchloric acid:
Perchloric acid has the formula HClO₄ and is a mineral acid. This colorless chemical, which is usually found as an aqueous solution, is a stronger acid than sulfuric acid, nitric acid, and hydrochloric acid.
When heated, it is a potent oxidant, however, at ambient temperature, aqueous solutions up to about 70% by weight are normally harmless, with only strong acid characteristics and no oxidizing properties. Perchloric acid is used to make perchlorate salts, particularly ammonium perchlorate, which is a key component of rocket fuel.
Perchloric acid is extremely corrosive and can easily combine with other chemicals to create explosive combinations.
Summary:
Perchloric acid has the formula HClO₄ and is a mineral acid. When heated, it is a potent oxidant, yet at room temperature, aqueous solutions up to about 70% by weight are normally safe for human ingestion.
 Production of Perchloric acid:
Production of Perchloric acid:
Perchloric acid is manufactured in two ways in the industrial world. The classic approach takes advantage of sodium perchlorate’s high aqueous solubility (209 g/100 mL water at room temperature). When such solutions are treated with hydrochloric acid, perchloric acid is produced, resulting in the precipitation of solid sodium chloride:
NaClO4 + HCl → NaCl + HClO4
Distillation can purify the concentrated acid. Anodic oxidation of aqueous chlorine at a platinum electrode is an alternate technique that is more direct and avoids salts.
 Laboratory preparations of Perchloric acid:
Laboratory preparations of Perchloric acid:
When barium perchlorate is treated with sulfuric acid, barium sulfate is precipitated, leaving perchloric acid. It can also be created by heating a mixture of nitric acid and ammonium perchlorate while adding hydrochloric acid.
Due to a parallel reaction involving the ammonium ion, the reaction produces nitrous oxide and perchloric acid, which can be concentrated and purified greatly by boiling out the leftover nitric and hydrochloric acids. Perchloric acid is commonly produced in one of two ways in the industrial world.
The first technique, also known as the classic way, is a method of making perchloric acid that makes use of sodium perchlorate’s extraordinarily high solubility in water. At room temperature, sodium perchlorate dissolves in water at a rate of 2090 grams per liter. \
When a solution of sodium perchlorate in water is treated with hydrochloric acid, perchloric acid is formed along with a precipitate of sodium chloride. This concentrated acid can also be purified by the distillation process. The second method includes the use of electrodes in which chlorine dissolved in water is anodically oxidized at a platinum electrode. However, the other procedure is thought to be more costly.
Summary:
Perchloric acid is commonly produced in one of two ways in the industrial world. The classic method takes advantage of sodium perchlorate’s high water solubility. Anodic oxidation of aqueous chlorine at a platinum electrode is an alternate approach that avoids the need for salts.
 Perchloric Acid formula:
Perchloric Acid formula:
The chemical formula for perchloric acid is HClO₄, and its molar mass is 100.46 g mol-1. A chlorine atom lies in the center of the acid structure, to which three oxygen atoms are bound by double bonds, and a fourth bond is created between the chlorine and a hydroxyl. This hydroxyl has an acidic proton, which gives the molecule its acidic property.
 A table about perchloric acid:
A table about perchloric acid:
| Perchloric acid formula | HClO₄ |
|---|---|
| Molar mass | 100.46 g/mol |
| Appearance | colorless liquid |
| Odor | odorless |
| Density | 1.768 g/cm3 |
| Melting point | −17 °C (1 °F; 256 K) |
| Boiling point | 203 °C (397 °F; 476 K) |
| Solubility in water | miscible |
| Acidity | −15.2 (±2.0); ≈ −10 |
| Conjugate base | Perchlorate |
 Properties of Perchloric acid:
Properties of Perchloric acid:
At room temperature, anhydrous perchloric acid is an oily, unstable liquid. It generates at least five hydrates, some of which have been crystallographically identified. The perchlorate anion is connected to H2O and H3O+ centers via hydrogen bonds in these solids. With water, perchloric acid forms an azeotrope, which contains around 72.5 percent perchloric acid.
This version of the acid is commercially available and has an indefinite shelf life. These types of solutions are hygroscopic. Concentrated perchloric acid dilutes itself by absorbing water from the air if left open to the air. Perchloric acid was once a colorless, odorless 70 percent water solution.
It has a density of 1.768 g mL-1 and is miscible in water. It has a melting point of -17 °C and a boiling point of 203 °C. With water, it can produce azeotrope. Perchloric acid is a strong acid with a pKa of -10, which indicates that the conjugated base is stable and practically entirely dissociated in aqueous solutions.
Fraude’s reagent, commonly known as perchloric acid, is a colorless, fuming, hygroscopic liquid that boils at 16°C (61OF). It is a powerful oxidant that is water-soluble. When cold dilute perchloric acid combines with metals like zinc and iron, hydrogen gas and metallic perchlorate are produced. Electrolytic baths, electropolishing, explosives, analytical chemistry, and medicine all use perchloric acid.
Perchloric acid is a colorless, flammable, oily liquid that is miscible with water and volatile at low pressure. When the concentration of HClO4 in H2O reaches 73 percent, a maximum constant-boiling solution (203 °C, 760 millimeters Hg) is obtained. When cold dilute perchloric acid combines with metals like zinc and iron, hydrogen gas is produced, as well as the perchlorate in solution.
From the standpoint of oxidation and reduction, it is stable (except that iodine is oxidized to periodic acid, with the liberation of chlorine, ferrous salt solutions to ferric). Concentrated hot perchloric acid, on the other hand, is a powerful oxidizing agent that explodes violently when it comes into contact with charcoal, paper, or alcohol; it can also cause significant skin wounds. Ammonium perchlorate is made by distilling it with HNO3 and HCl.
Summary:
Fraude’s reagent, or perchloric acid, is a colorless, fuming, hygroscopic liquid that boils at 16°C. Electrolytic baths, electropolishing, explosives, analytical chemistry, and medicine are all applications for it.
 Uses of Perchloric acid:
Uses of Perchloric acid:
Perchloric acid is primarily manufactured as a precursor to ammonium perchlorate, a rocket fuel. Perchloric acid manufacturing has increased as rocketry has grown in popularity. Annually, many million kg are generated.
Perchloric acid has unique features in analytical chemistry and is one of the most proven materials for etching liquid crystal displays and essential electronics applications, as well as mineral extraction. It is also beneficial in the etching of chromium. Perchloric acid is most commonly used as a precursor to ammonium perchlorate, an inorganic chemical that is an important component of rocket fuel.
As a result, perchloric acid is regarded as a critical chemical molecule in the space industry. This substance is also used to etch liquid crystal display devices (often abbreviated to LCD). Perchloric acid is therefore commonly employed in the electronics industry. Because of its unusual characteristics, this molecule is also employed in analytical chemistry.
Perchloric acid also has a variety of uses in the extraction of materials from their ores. In addition, this substance is employed in the etching of chrome. Perchloric acid is one of the strongest Bronsted-Lowry acids because it works as a superacid.
 As an acid:
As an acid:
One of the strongest Bronsted–Lowry acids is perchloric acid, which is a superacid. The fact that its monohydrate contains distinct hydronium ions and can be separated as a stable, crystalline solid, formulated as [H3O+][ClO– 4], indicates that its pKa is less than 9.
Its aqueous pKa is now estimated to be −15.2±2.0. Because perchlorate is a weak nucleophile, it offers strong acidity with minimal interference (explaining the high acidity of HClO4). Other noncoordinating anions acids, such as fluoroboric acid and hexafluorophosphoric acid, can be hydrolyzed, but not perchloric acid.
Despite the dangers of its salts’ explosiveness, the acid is frequently used in specific syntheses. It’s also a good eluent for ion-exchange chromatography for identical reasons. Electropolishing and etching of aluminum, molybdenum and other metals are also done using it.
The following are a few examples of perchloric acid’s applications:
• In the separation of sodium and potassium, perchloric acid is utilized as an oxidant.
• Used in the manufacture of explosives.
• Used for metal plating.
• 1H-Benzotriazole is determined with this reagent.
• It’s a catalyst.
• It’s a component of rocket fuel.
• Used for molybdenum electropolishing and etching.
Summary:
Perchloric acid is primarily manufactured as a precursor to ammonium perchlorate, a rocket fuel. Electropolishing and etching of aluminium, molybdenum, and other metals are also done using it. Annually, many million kg are generated.
 Safety of Perchloric acid:
Safety of Perchloric acid:
Perchloric acid is heavily regulated due to its powerful oxidizing effects. It reacts violently with metals (such as aluminium) and biological materials (wood, plastics). To avoid the accumulation of oxidizers in the ductwork, work with perchloric acid must be done in fume hoods with a wash-down capability.
On February 20, 1947, a bath containing approximately 1000 liters of 75 percent perchloric acid and 25 percent acetic anhydride by volume burst in Los Angeles, California, killing 17 persons and injuring 150 more. The O’Connor Electro-Plating plant was destroyed, along with 25 other buildings and 40 autos, and 250 surrounding residences were damaged.
Electro-polishing aluminium furniture was done in the bath. When an iron rack was replaced with one covered with cellulose acetobutyrate, organic compounds were added to the heated bath. The bath exploded a few minutes later.
Summary:
Perchloric acid is a powerful oxidizer. It reacts violently with metals and organic materials. To avoid the accumulation of oxidizers in ductwork, work with it must be done in fume hoods with wash-down capabilities.
 Health Hazard of Perchloric acid:
Health Hazard of Perchloric acid:
Inhalation, ingestion, or skin or eye contact with fumes or substances can result in serious harm, burns, or death. Gases that are unpleasant, caustic, and/or toxic may be produced by a fire. Pollution may be caused by runoff from firefighting or diluting water.
Perchloric acid in concentrated form is a highly corrosive material that can cause skin burns when it comes into touch with it. It irritates the eyes and mucous membranes severely. This chemical has moderate toxicity. Excitation, a drop in body temperature, and difficulty breathing are some of the hazardous signs of consumption.
Perchloric acid is a very corrosive chemical that produces severe burns to the eyes, skin, and mucous membranes when it comes into contact with them. Perchloric acid has mild acute toxicity. The eyes, mucous membranes, and upper respiratory tract are all severely irritated by this chemical. Perchlorates irritate the body wherever they come into contact with it. In humans, perchloric acid is not carcinogenic or causes reproductive or developmental harm.
 Fire Hazard of Perchloric acid:
Fire Hazard of Perchloric acid:
In the event of a fire, these compounds will hasten the burning process. When heated or involved in a fire, some may degrade explosively. Heat or pollution may cause it to explode. With hydrocarbons, some will react explosively (fuels). It has the potential to ignite combustibles (wood, paper, oil, clothing, etc.).
When heated, containers have the potential to explode. Runoff can cause a fire or other hazards. Perchloric acid is a flammable substance. Anhydrous (dehydrated) acid poses a significant explosive risk. It’s unstable, and it can break down explosively at room temperature or when it comes into touch with a variety of organic chemicals.
Ammonium, alkali metal, an alkali earth perchlorates are less dangerous than heavy metal perchlorates and organic perchlorate salts. Perchlorate mixtures with a variety of oxidizable chemicals are explosive.
 Flammability and Explosibility of Perchloric acid:
Flammability and Explosibility of Perchloric acid:
Perchloric acid is a flammable substance. Anhydrous (dehydrated) acid poses a significant explosive risk. It’s unstable, and it can break down explosively at room temperature or when it comes into touch with a variety of organic chemicals.
Ammonium, alkali metal, an alkali earth perchlorates are less dangerous than heavy metal perchlorates and organic perchlorate salts. Perchlorate mixtures with a variety of oxidizable chemicals are explosive.
Summary:
Perchloric acid is a very corrosive chemical that produces severe burns to the eyes, skin, and mucous membranes when it comes into contact with them. Anhydrous (dehydrated) acid poses a significant explosive risk. Perchlorate mixtures with a variety of oxidizable chemicals are explosive.
 What is an acid?
What is an acid?
An acid is a molecule or ion capable of donating a proton (i.e., hydrogen ion, H+) or creating a covalent bond with an electron pair, known as a Bronsted–Lowry acid or a Lewis acid. The proton donors, also known as Bronsted–Lowry acids, are the first group of acids.
Proton donors, which generate the hydronium ion H3O+ in aqueous solutions, are known as Arrhenius acids. The Arrhenius theory was expanded by Bronsted and Lowry to include non-aqueous solvents. A hydrogen atom is frequently attached to a chemical structure that is still energetically advantageous following the loss of H+ in a Bronsted or Arrhenius acid.
Characteristic features of aqueous Arrhenius acids provide a practical description of an acid. Acids produce sour aqueous solutions, can turn blue litmus red, and form salts when they combine with bases and certain metals (such as calcium). The word acid comes from the Latin acidus/acre, which means sour.
While the technical definition applies only to the solute, an aqueous solution of an acid with a pH less than 7 is also referred to as “acid” (as in “dissolved in acid”). A lower pH indicates that the solution is more acidic, resulting in a higher concentration of positive hydrogen ions in the solution.
Acidic chemicals or compounds are those that have the property of an acid. Hydrochloric acid (a hydrogen chloride solution found in gastric acid in the stomach that activates digestive enzymes), acetic acid (vinegar is a dilute aqueous solution of this liquid), sulfuric acid (used in vehicle batteries), and citric acid are all examples of aqueous acids (found in citrus fruits).
Acids (in the colloquial sense) can be solutions or pure substances, and they can be generated from acids (in the precise definition) that are solids, liquids, or gases, as these instances demonstrate. Strong acids and some concentrated weak acids are corrosive, however, carboranes and boric acid are exceptions.
Lewis acids are the second type of acid, as they create a covalent bond with an electron pair. Boron trifluoride (BF3), for example, contains an empty orbital on the boron atom that can form a covalent bond by sharing a lone pair of electrons with an atom in a base, such as the nitrogen atom in ammonia (NH3).
As a generalization of the Bronsted definition, Lewis defined an acid as a chemical species that accepts electron pairs directly or indirectly by releasing protons (H+) into the solution, which subsequently accept electron pairs. Hydrogen chloride, acetic acid, and most other Bronsted–Lowry acids, on the other hand, are not Lewis acids because they cannot form a covalent bond with an electron pair.
Many Lewis acids, on the other hand, are not Arrhenius or Bronsted–Lowry acids. Because scientists generally always refer to a Lewis acid explicitly as a Lewis acid, an acid is implicitly a Bronsted acid rather than a Lewis acid in modern nomenclature.
Summary:
A Bronsted–Lowry acid is a molecule or ion capable of giving a proton (i.e., the hydrogen ion, H+). Acids produce sour aqueous solutions, can turn blue litmus red, and react with bases to produce salts.
 Related compounds to Perchloric acid:
Related compounds to Perchloric acid:
 1- Hydrochloric acid:
1- Hydrochloric acid:
Muriatic acid, also known as hydrochloric acid [H+(aq) Cl−(aq) or H3O+ Cl−], is an aqueous solution of hydrogen chloride (chemical formula HCl). It’s a colorless liquid with a strong, pungent odor. It’s considered a strong acid. In the digestive processes of most animal species, including humans, it is a component of stomach acid. Hydrochloric acid is a common laboratory reagent and chemical used in industry.
 2- Hypochlorous acid:
2- Hypochlorous acid:
When chlorine dissolves in water, hypochlorous acid (HOCl or HClO) develops, which then partially dissociates to generate hypochlorite. Oxidizers HClO and ClO are the principal disinfection agents in chlorine solutions. Due to quick equilibration with its precursor, HClO cannot be separated from these solutions.
Bleaches, deodorants, and disinfectants are sodium hypochlorite (NaClO) and calcium hypochlorite (Ca(ClO)2). Hypochlorous acid is naturally found in the white blood cells of mammals, including humans. It is non-toxic and has been used for many years as a safe wound treatment solution. Hypochlorous acid water has been proven to have strong disinfectant effects when dissolved in water.
It has been recognized as a valuable cleaning agent and sanitizer due to this and its non-toxicity. It has been identified as a disinfectant efficient against COVID-19 by the US Environmental Protection Agency, which is backed up by clinical investigations. It’s also utilized as a commercial deodorizer because of its ability to permeate pathogen membranes.
 3-Chlorous acid:
3-Chlorous acid:
Chlorous acid has the formula HClO2 and is an inorganic chemical. It’s a very weak acid. In this acid, chlorine has an oxidation state of +3. Although the acid is difficult to obtain in pure form, the conjugate base formed from it, chlorite, is extremely stable. The well-known sodium chlorite is an example of an anion salt. This salt, along with others, is sometimes used to make chlorine dioxide.
 4- Chloric acid:
4- Chloric acid:
The formal precursor of chlorate salts is chloric acid (HClO3), a chlorine oxoacid. It is an oxidizing agent and a strong acid (pKa 2.7). In terms of disproportionation, chloric acid is thermodynamically unstable. Chloric acid is stable in cold aqueous solution up to a concentration of around 30%, and a solution with a concentration of up to 40% can be made by careful evaporation under reduced pressure.
Summary:
Hydrochloric acid is a common laboratory reagent and chemical used in industry. It’s a colorless hydrogen chloride solution with a pronounced unpleasant odor. Hypochlorous acid is a naturally occurring substance found in the white blood cells of mammals, including humans.
 Is perchloric acid dangerous?
Is perchloric acid dangerous?
Perchloric acid is a highly effective oxidant. This chemical has extremely high reactivity towards most metals due to its strong oxidizing characteristics. In addition, this chemical is extremely reactive with organic materials. This substance has the potential to be corrosive to the skin. As a result, sufficient safety precautions must be used when handling this chemical.
 Why is Perchloric Acid Considered to Be the Strongest Acid?
Why is Perchloric Acid Considered to Be the Strongest Acid?
Perchloric acid is HClO4. H+ is connected to one oxygen atom, which forms a single bond with chlorine, and the remaining three oxygen atoms create a coordinate bond with chlorine. If a substance produces proton in an aqueous solution, it is considered to be acid, whereas if its conjugate base is stable, it is said to be a strong acid.
In that situation, the stability of the perchlorate ion is due to the negative charge conjugation that has been established on the oxygen atom, as well as the three other oxygen atoms.
 Perchloric acid in the laboratory:
Perchloric acid in the laboratory:
Perchloric acid (HClO4) is a colorless, clear liquid that can be used as a strong oxidant in the laboratory. Perchloric acid is useful in chemical processing because it has the properties of a mineral acid without the addition of ions like chloride, nitrate, or sulphate.
This corrosive substance has the same risks as most acids: it can cause digestive and respiratory system burns if consumed, and it can cause eye and skin burns if exposed to the exterior of your body. Perchloric acid is also explosively unstable under certain circumstances. In solution, perchloric acid is not explosive, but it is exceedingly corrosive and hazardous to inhale. This is a good reason to utilize a chemical fume hood.
When perchloric acid is evacuated through the same ventilation system that collects organics, the salt residue of the acid is saturated by the organics, and a new, very unstable molecular structure is produced. When the perchloric acid vapor is allowed to condense in the ductwork and subsequently evaporate, a salt called perchlorate is left behind.
Heat, flame, friction, percussion, or a chemical reaction can all be used to detonate perchlorate crystals. Though anything as minor as the vibration of the blower motor can create a dramatic reaction, there are usually no further issues until the system is dismantled. The risk arises when a mechanical contractor, uninformed of the dangers present, tries to dismantle or service the mechanical system, dislodging crystals in the process, leading to a disastrous situation.
Perchloric acid, fortunately, may be neutralized by water, and perchlorate salts can be dissolved in water. Perchloric acid applications necessitate specialized equipment such as complete washdown systems, unique construction materials, and specialized mechanical systems.
There may be exceptions to the above, particularly if the acid is dilute, used in small amounts, and not heated. In this case, extreme caution should be exercised to avoid spills. Perchloric transfers are an example of this type of activity. To assess if the specific application falls under this category, the matter should be discussed with the facility’s Health and Safety Officer.
Summary:
If swallowed or inhaled, perchloric acid is very corrosive and dangerous to one’s health. If introduced to the exterior of the body, it can cause eye and skin burns. When the chemical is evacuated in a chemical fume hood, it can be explosive under specific conditions.
 Safe Handling of Perchloric acid:
Safe Handling of Perchloric acid:
• When handling perchloric acid, wear proper Personal Protective Equipment (lab coat, safety glasses, and acid-resistant gloves).
• Never handle perchloric acid on a wooden surface, and keep it away from oxidizable materials like clothes, paper towels, or grease. After absorbing perchloric acid liquid or vapor, such things can become very combustible and may spontaneously burn or even explode.
• Perchloric acid should not be exposed to extreme dehydration.
• Dilute by mixing perchloric acid with water rather than acid with water.
• If perchloric acid solutions are filtered through a paper filter, the filter (and precipitate) should be thoroughly cleaned with water to eliminate all perchlorate before drying.
• If temperatures are likely to increase above ambient levels, DO NOT combine concentrated perchloric acid (>72 percent) with organic compounds.
• Perchloric acid digestions and other high-temperature applications necessitate the employment of a specially built fume hood with a water wash-down system. This technology is essential to prevent explosive perchlorates from accumulating in the ducting.
• Do not use an oil bath to heat perchloric acid. A sand bath, a heating mantle, or a hot plate can all be used.
Summary:
To avoid the development of explosive perchlorates in the ductwork, perchloric acid digestions and other applications at extreme temperatures must be carried out in a specially built fume hood with a water wash-down system.
 Frequently Asked Questions:
Frequently Asked Questions:
The following are some of the most frequently asked questions concerning this keyword:
 1- What is perchloric acid used for?
1- What is perchloric acid used for?
Perchloric acid is a powerful acid that is used to decompose organic matter completely. It’s usually sold in 70-72 percent strength bottles with a capacity of up to one gallon. Its dangers are similar to those of nitric acid in many ways, as both are potent oxidants.
 2- Is perchloric acid the strongest acid?
2- Is perchloric acid the strongest acid?
One of the most powerful mineral acids is perchloric acid (HClO₄). Perchloric acid, when heated and concentrated, has strong oxidizing and dehydrating capabilities, and it reacts violently with organic molecules.
 3- What does perchloric acid do to the skin?
3- What does perchloric acid do to the skin?
Perchloric acid, up to 72 percent concentrations, has characteristics similar to other strong mineral acids at ambient temperature. It is a very corrosive material that produces severe burns to the eyes, skin, and mucous membranes when it comes into contact with them. Perchloric acid reacts as a powerful non-oxidizing acid when employed in these conditions.
 4- Why is a perchloric acid a very strong acid?
4- Why is a perchloric acid a very strong acid?
Perchloric acid has three double bonds whereas sulfuric acid only has two. As a result, perchloric acid’s conjugate base is more stable, implying that the acid is more acidic. When both are put to water, they ionize, making them identical in strength in an aqueous medium.
 5- Is perchloric acid harmful?
5- Is perchloric acid harmful?
Yes. When consumed, perchloric acid causes digestive and respiratory system burns, and when exposed to the outside of the body, it causes eye and skin burns.
 6- What is the deadliest acid?
6- What is the deadliest acid?
Fluoroantimonic acid, HSbF6, is the world’s most powerful superacid. It’s made by combining hydrogen fluoride (HF) with antimony pentafluoride (SbF5). The superacid can be made in a variety of ways, but the strongest superacid known to man is made by mixing equal parts of the two acids.
 7- What’s the difference between hydrochloric acid and perchloric acid?
7- What’s the difference between hydrochloric acid and perchloric acid?
Perchloric acid differs from hydrochloric acid in that it contains hydrogen, chlorine, and oxygen atoms, whereas hydrochloric acid solely has hydrogen and chlorine atoms. Because of their high acidic nature, perchloric and hydrochloric acids are crucial in chemical synthesis reactions.
 8- How long is perchloric acid good for?
8- How long is perchloric acid good for?
It is not recommended to store anhydrous perchloric acid. Short-term storage, even less than 10 days, poses a serious risk.
 9- Is perchloric acid a weak acid?
9- Is perchloric acid a weak acid?
All of the other acids are weak. Hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid are strong acids. Hydrofluoric acid is the only weak acid generated when hydrogen reacts with a halogen (HF).
 10- What is the oxidation number of perchloric acid?
10- What is the oxidation number of perchloric acid?
HClO₄ is the formula for perchloric acid and the oxidation number of perchloric acid is +1.
Conclusion:
HClO₄ is the formula for perchloric acid. Perchloric acid has the formula HClO₄ and is a mineral acid. This colorless chemical, which is usually found as an aqueous solution, is a stronger acid than sulfuric acid, nitric acid, and hydrochloric acid. Perchloric acid is a colorless, flammable, and hygroscopic liquid that boils at 16 degrees Celsius.
Electrolytic baths, electropolishing, explosives, analytical chemistry, and medicine are all applications for it. Perchloric acid is primarily manufactured as a precursor to ammonium perchlorate, a rocket fuel. Electropolishing and etching of aluminium, molybdenum, and other metals are also done using it. Annually, many million kg are generated.
 Related Articles:
Related Articles:
![]() Strong Acids
Strong Acids
![]() Sulfurous Acid
Sulfurous Acid
![]() Phosphoric Acid Formula
Phosphoric Acid Formula
![]() Aluminum Dichromate Formula
Aluminum Dichromate Formula