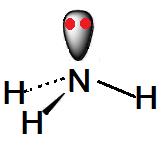
The formula of ammonia is NH3. it’s a liquid which has the molecular mass (17 amu). The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom within the middle, with the one lone pair of electrons on top of the atom.
What is the Lewis structure of NH3?
why ammonia acts as a Lewis base, because it can donate those electrons. The (NH3) molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory (VSEPR theory) with an experimentally determined bond angle of 106.7°.
Arrangement of atoms in Nh3
The central nitrogen atom has five outer electrons with an extra electron from each H atom. The ammonia molecule has the structure of like a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Ammonia has 4 regions of electron density on all sides of central nitrogen atom (3 bonds and one lone pair). These are arranged in a tetrahedral shape. The resulting molecular shape or structure is like trigonal pyramidal with H-N-H angles of 106.7°. Ammonia contains a trigonal pyramidal or distorted tetrahedral structure because of the repulsive lone pair – bond pair interaction. Also, the bond angle in ammonia is a smaller amount than standard 109′ because of a similar reason.
The bond angle is 107. ammonia has one lone pair because the nitrogen is just forming 3 bonds, one in every of the pairs must be a lone pair ,due to this, there’s more repulsion between a lone pair and a bonding pair than there’s between two bonding pairs. That forces the bonding pairs together slightly - reduce the bond angle from 109.5° to 107°.
In Ammonia or (NH3) the central atom which is nitrogen is sp3 hybridized. It didn’t really go anywhere, the lone pair on nitrogen in ammonia pickes up a proton and forms a covalent bond.
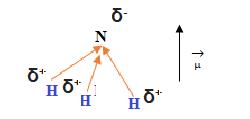
The consequence of this is often now there’s another proton than electrons within the molecule so it’s a positive charge. Ammonia is a nucleophile because it’s a lone pair of electrons and a δ⁻ charge on the N atom. … Ammonia doesn’t carry a negative charge.
But it’s a lone pair of electrons. And nitrogen is more electronegative than hydrogen, therefore the nitrogen atom contains a δ⁻ charge.
NH3 lewis structure molecular geometry:
Now let’s move forward and realize the electron geometry. NH3 electron geometry is: ‘Tetrahedral,’ because it has four group of electrons. One group has an unshared pair of electrons. ‘N’ has tetrahedral electronic geometry.
Thus, Ammonia is an example of the molecule during which the central atom has shared as well as an unshared pair of electrons. So, that’s all for the Ammonia. I hope I actually have given the data of Ammonia or NH3 you were expecting.
The Geometry of Molecules is an amazingly compelling and exciting subject and to understand such basics is important if you’re entering in the real chemistry field. Stay curious always and check out to identify each aspect by your own with the logic and magic of science.
H2O lewis structure:
Lewis structure of water molecule contains 2 single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are wont to draw lewis structure. in the lewis structure of water molecule, there are two single bonds around oxygen atom. Hydrogen (H) atoms are ■■■■■ to oxygen (O) atom through single bonds.
Also, there are 2 lone pairs on oxygen (O) atom. Water molecule may be a simple molecule. Drawing lewis structure of water molecule is easy than a number of other complex molecules or ions. Imagine drawing lewis structure of thiosulfate ion. There are few steps to follow to draw a lewis structure properly.
The Lewis Structure for H2O is explained in below Following steps.
- Find the total number of electrons of the hydrogen atoms and the valence shells of the oxygen atom.
2.Total electron pairs as lone pairs and bonds
Central atom selection.
-
Mark the odd pairs in the atoms.
-
Mark charges on the atoms, if any.
-
Check the stability by converting single pairs into bonds and minimize the charges on the atoms to get the best lewis structure.
CH4 lewis structure:
In CH4, the central atom is a carbon. In the electron point structure, we represent the e-value of the element. Thus, the Carbon © electron (e) has 4 electrons in the point structure and one electron in the hydrogen (H) atom.
C - H share electrons to form a single bond. CH4 Lewis Structure Lewis Structure for CH4 (Methane). In the CH₄ (methane) Lewis Dot structure, we must first find the valence electrons of carbon and hydrogen. We express the valence electrons as points in the lewis point structure. To induce carbon’s valence electrons, we must appear in the electronic configuration of carbon.
C (6) = 1s²2s²2p²
Here the value of the main quantum number (n) is n = 2.
The highest value of the main quantum number, n, indicates the valence shell, and we know that the electrons in the valence shell are called the valence shell. The number of valence electrons in carbon is four.
CO2 lewis structure:
In the formation of CO2, there are two particles; Carbon and oxygen. Carbon is in group 4 and oxygen in group 6. In addition, there are 2 oxygen. So CO2 = 4 + 6 (2) = 16. So the total valence electrons are 16.
Carbon is the least electronegative, which means it stays in the middle. So put the carbon in the middle and set the oxygen on either side of that. Now let’s check and see if we have any bytes. The oxygen on your right has 8. The oxygen on your left has 8. So they both have bytes. and also carbon has only 4 valence electrons; it has no bytes.
it’s time to share those unbound electrons between the two atoms! it will look like this. Start by considering the oxygen atom. As you will see, oxygen has 8 electrons. Then it’s perfect. and also carbon a 6; which is a bit closer. Now repeat a process similar to the other oxygen electron. Let’s take a few electrons and share them on the other side so that oxygen can have 8 and carbon 6.
Hybridization of CO2
The hybridization of CO2. To understand it, we have to look at every carbon atom. Observe each region around the particles to learn more about the hybridization of the CO2 molecules.
If we start with the carbon atom, there are two double bonds. So there is nothing wrong with saying that there is a sigma bond on all sides and a PI bond above it.
There are no unbonded electron pairs on the central carbon, so there are only two sigma bonds. There are two regions; which means that there are S and a P orbital hybridizing. Hence the hybridization on carbon is “SP”.
If we talk about the oxygen atoms, since they are symmetrical, we only need to study one atom. If you watch closely, there is a sigma bond on the right side of the oxygen. You will also find two unbound electron pairs.
So we will say that three regions are bound to the oxygen, which is what makes hybridization - SP2 - happen. In this case, since all the oxygen atoms are similar, the others are similar to the other current ones. So this is the hybridization of CO2.