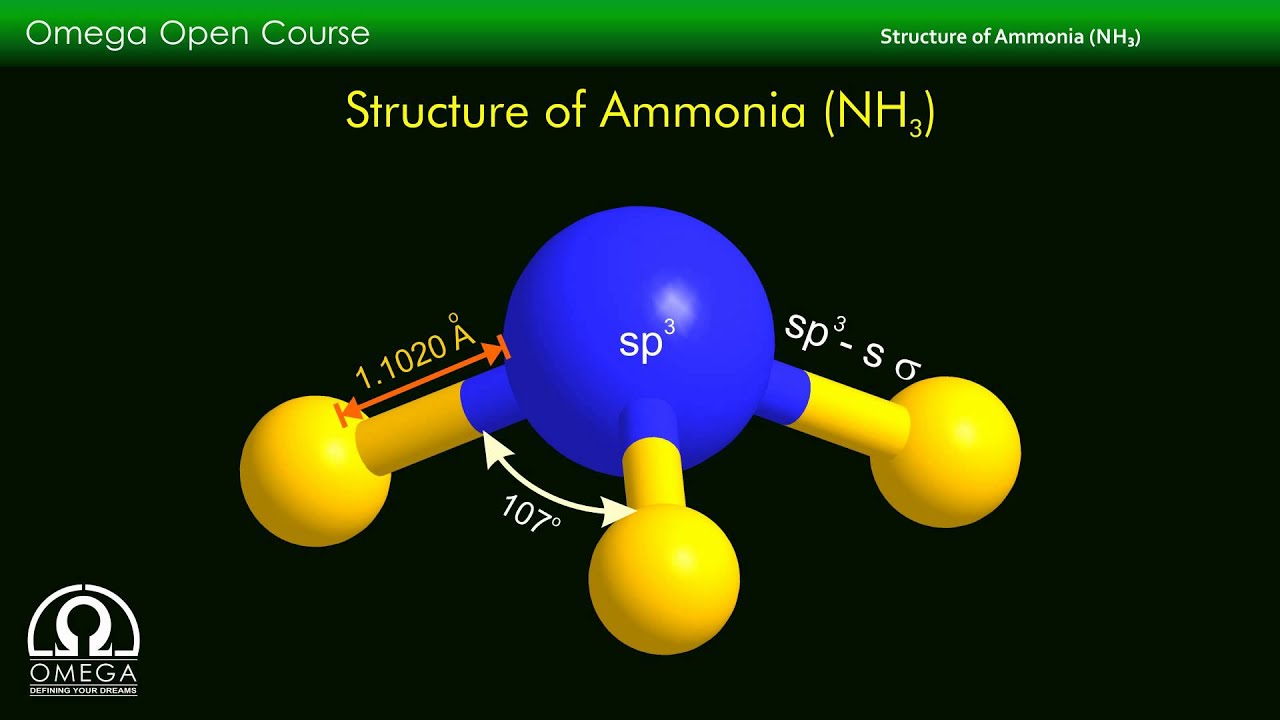
nh3 molecule has a tetrahedral molecular structure, similar to water. The nonpolar molecule is symmetrically arranged around the nitrogen base, protecting it from the elements.
 nh3 molecule
nh3 molecule
We may learn about electron geometry, polarity, and other polar and nonpolar compound features via Lewis structure. The shape of NH3 molecules is determined by the pair of electrons that form bonds. We must first select the valence electrons that form dipole moments before we can get to the structure of Ammonia molecules.
 Structure of the NH3 Molecule
Structure of the NH3 Molecule
| Name of molecule | Ammonia (NH3) |
|---|---|
| Bond Angles | 107.3 degrees |
| Molecular Geometry of NH3 | Pyramidal planar |
| Hybridization of NH3 | SP 3 hybridization |
| Electronic configuration | 1S22S22P3 |
There are five valence electrons in the outer shell of Nitrogen, whereas Hydrogen atoms only have one, although there are three with a concentrator in this group. As a result, we increase the number of valence atoms in our molecule by three.
The trigonal pyramidal form is the overall shape of NH3 because we now know the net dipole moment set by the entire valence electron. At its centre lies Nitrogen, which has an uneven charge distribution across three bonds and one lone pair, giving rise to the Trigonal Pyramidal structural design.
 Is Ammonia Nonpolar Or Polar?
Is Ammonia Nonpolar Or Polar?
Lewis structure, electron geometries, and molecular geometry of this molecule had an enormous impact on the out experiment outcome. You look at the chemical formula for Ammonia; you’ll see it has polar molecules.
Asymmetric molecules with polar bonds were formed when two atoms had chemical connections. Animal waste products are the two primary sources of this polar molecule: vegetable matter and nitrogenous. Small amounts of ammonia and ammonium salts are detected. However, when rain falls.
 Geometry
Geometry
Ammonia has a tetrahedral molecular structure, as previously stated. The nitrogen base is covered in a symmetrical arrangement of the nonpolar molecule. TheThe two nonpolar molecules generated molecular geometry of NH3 trigonal pyramidal.
Consider the distorted shape as resulting from single pair of electrons, which causes bonding pairs to repel each other. Due to the lone team on the Rogen atom, the bond angle should have been 109.5 degrees for trigonal pyramidal molecular geometry.
 Electronegativity
Electronegativity
The primary defining characteristic of a covalent molecule is the difference in electronegativity. It’s the same with nitrogen atoms: the more electronegative they are, the stronger the polarity of these covalent connections.
 Dipole
Dipole
The dipole moment of the product of the atom’s charge and the distance between them. This bond’s dipole moment will be from H to N. Three N-H bonds have a net dipole moment of 1.4 D. 3.335641034 C m = 1 D, as we have previously learned.
With the symmetrical form of the polar molecule, dipole moments in NH3 are calculated to be around 1.46D. Polar bonds join the atoms of these chemical compounds, resulting in asymmetrical molecules.
Summary
Colourless and odourless, Ammonia (NH3) is a stable hydride consisting of one Nitrogen atom, three hydrogen atoms, and three oxygen atoms. In addition to its unpleasant smell, Ammonia contains fertilizer and nitrogenous components, making it a helpful chemical.
 Ammonia Gas
Ammonia Gas
Ammonia is a colourless, toxic gas with a strong odour that is well-known. Anaerobic degradation of plant and animal matter is the primary source; it has around in space. Using rhizobia bacteria and plants, some most primarily games make Ammonia by “fixing” atmospheric Nitrogen.
Since the dawn of time, the pungent smell of Ammonia has been well-documented care. Wilhelm Scheele (Sweden/Germany), Peter Woulfe (Ireland), and Joseph Priestley (Scotland) are some of the world’s foremost outstanding miss (England).
Claude Louis Berthollet, a French chemist, identified its elements in 1785. Nitto made Ammonia and nitrogen hydrogen are combined in a high-temperature, high-pressure process tohen Fritz Haber and Carl Bosch came up with the idea for the procedure in 1909.
It was a German invention. Their work was recognized with the Nobel Prize in Chemistry, but Haber won in 1918,d Bosch won in 1931, two decades apart. It is still possible to employ the basic Haber–Bosch process today.
 Major Sectors Of Ammonia
Major Sectors Of Ammonia
Two thousand two hundred forty-two hundred twenty-four lion metric tonnes of Ammonia will be produced in the world 2020. (Mt). The actual output was 187 million tonnes. It is the world’s tenth most manufactured chemical.
85 to Eighty-five-cent of the world’s ammonia production is used in agriculture, either directly or indirectly. Other nitrates and urea are examples of chemical fertilizers generated by using Ammonia.
WatThe water utility of Ammonia varies with temperature, but it is generally very high (see fast facts). Ammonium hydroxide, known as aqueous Ammonia cannot be isolated. It is called “ammine” when Ammonia is employed as a ligand in coordination complexes.
Despite its widespread use in agriculture, Ammonia is currently manufactured using hydrogen obtained from fossil fuels and is the “green” product.
If hydrogen is produced by other methods such as the electrolysis of water powered by wind or solar energy, environmentally friendly Ammonia may be on the horizon.
 Properties - Chemical and Physical
Properties - Chemical and Physical
Ammonia (NH3) is a widely used industrial chemical in the United States. Ammonia is a nitrogen compound. As a result, it is used in business and commerce as man bodies and the natural world. Biochemical reactions require Ammonia, a precursor for synthesizing into acids and nucleotides.
Ammonia has the following chemical and physical properties:
Irritating, colourless, and unpleasant at room temperature are only some ammonia characteristics Anhydrous. Ammonia is hygroscopic in its purest form (readily absorbs moisture).
Ammonia is corrosive due to its alkaline characteristics.
As the gas dissolves in water, ammonium hydroxide is formed. This cauacidiclution and a weak base are highly corrosive.
-
Compression of ammonia gas results in the formation of a transparent liquid.
-
Steel containers are commonly used to convey Ammonia as a compressed liquid.
-
Containers of Ammonia can explode if they are exposed to high temperatures.
Inhalation of ammonia gas or vapours is the most common met exposure method for most people to use. Exposure to Ammonia can arise from various sources, including the natural world and household cleaning products.
On Exposure can occur either accidentally or intentionally norms and in industrial and commercial areas where Ammonia is commonly used; e However, liquid anhydrous ammonia gas generates heavier vapours when exposed to moisture. PeoIfey spread along the ground or into low-lying locations with limited airflow.
Summary
Ammonia is a component of the nitrogen cycle mandated in soil by bacteria. Plant, animal, and animal waste degradation all contribute to the natural production of Ammonia. Anhydrous ammonia gas generally does not settle in low-lying locations because it is lighter than air.
 Use Of Ammonia
Use Of Ammonia
It’s no secret that Ammonia is used to produce many everyday items. It’s a colourless gas with a distinct odour. Humans, other animals, and the rest of the natural world are exposed to it.
For the human body to produce Ammonia, it must break down protein-rich foods like meat into amino acids convert the ammonium into urea.
 An Ammonia-Based Fertilizer
An Ammonia-Based Fertilizer
Fertilizers like ammonium nitrate, which releases nitrogen into the soil to help plants develop, start with Ammonia as a building ingredient. The vast majority of Ammonia produced worldwide is utilized in fertilizer, which helps feed billions of people.
Soil nutrient reserves are naturally depleted due to the cultivation of food crops. Farmers rely on fertilizer to make their soils productive to produce healthy harvests. Supplementing food crops with nutrients like zinc, selenium, and boron are possible through fertilizers.
 Ammonia in Cleaning Agents
Ammonia in Cleaning Agents
Household ammonia, or ammonium hydroxide, is an ingredient in many household cleaning solutions used to clean tubs, sinks, toilets, counters and tile surfaces.
Cooking grease and wine stains are easily removed with Ammonia, as can other home dirt and stains caused by animal fats or vegetable oils. It is usual to use Ammonia in glass cleaning products because it quickly evaporates.
 Industrial/Manufacturing Ammonia Uses
Industrial/Manufacturing Ammonia Uses
In addition to purifying water sources, Ammonia can be utilized to produce a wide range of products, such as polymers, explosives and textiles.
Aside from being a stabilizer and neutralizer, it is also used as a nitrogen source in the food and beverage industries. In air-conditioning systems and as refrigerant gas, Ammonia can absorb a lot of heat from the environment.
 Agricultural use of Ammonia
Agricultural use of Ammonia
More than half of the world’s food supply is dependent on the use of mineral fertilizers. There has been a steady increase in fertilizer use as the world population has grown and people have shifted their diets to include more meat.
Ammonia is a significant player in this equation. Nitrogen fertilizer manufacturing is made possible because Ammonia binds airborne Nitrogen and makes it available for nitrogen fertilizer manufacture.
 Agricultural applications of Ammonia
Agricultural applications of Ammonia
Plants need nutrients like Nitrogen, potassium, phosphorus, magnesium, sulphur, and calcium to thrive and produce fruit. As with humans, each plant is unique and requires a precise amount of nutrition to thrive, based on the sort of plant it is and where it grows.
-
Crops may need between 20 and 350 kilos of fertilizers per hectare throughout their growth.
-
Due to various reasons, however, the soil often lacks the nutrients necessary for plant growth.
-
Either they don’t naturally occur in large enough amounts, or they aren’t available for purchase.
-
Leaching or harvesting removes them from the soil.
-
Fertilization alone can make up for the deficiency of plant nutrients and ensure future cultivation.
 Anti-overfertilization strategy
Anti-overfertilization strategy
Over-fertilization can hurt the soil, surface water, and groundwater because of the dispersion of mineral utilization and excess Nitrogen in the food supply. To avoid this, farmers must find ways to minimize their ecological impact without sacrificing any of the potential harvests they may have.
Farmers require a fertilizer whose nutrient composition and release profile are specifically tailored to meet the crop’s needs under the specific climatic and soil circumstances that they are working with.
For the fertilizer producer, this entails developing fertilizers that feed plants more efficiently while creating less waste and emissions during the application minimizing raw materials.
 Ammonium nitrate reduces emissions
Ammonium nitrate reduces emissions
Not only does environmental preservation play a part in the application of fertilizer, but it also does so in the manufacturing process. It’s always important to consider the amount of CO2 produced during the manufacturing process.
Based on green Ammonia, ammonium nitrate or calcium ammonium nitrate (CAN) fertilizer has one of the lowest carbon footprints of any available fertilizer per kilogramme of product and per kilogramme of Nitrogen given to the land.
Calcium is also good for the soil and plants. Protects plants from growing deformed leaves and buds by increasing plant size. As a bonus, calcium helps farmers protect their soil since it controls the pH and encourages the growth of microorganisms.
Summary
Fertilizer with a nitrogen content of 25 to 28 per cent, CAN is ideal for Europe’s acidic soil and colder temperatures. Quick-release Nitrogen makes Nitrogen in ammonium nitrate fertilizer rapidly available to plants, preventing soil exploitation.
 How does ammonia exposure affect health?
How does ammonia exposure affect health?
Irritating and destructive, Ammonia is not a good chemical to work with. The nose, throat, and respiratory system begins to burn immediately exposed to high amounts of Ammonia in the air.
Respiratory discomfort or failure can ensue from bronchiolar and alveolar oedema and airway. Coughing and a runny nose and throat can result from inhaling modest quantities of irritants.
At low concentrations, one may not be aware of their prolonged Exposure due to Ammonia’s olfactory fatigue or adaptation caused by its odour.
Children exposed to the same ammonia vapour levels as adults may receive a higher dose because of their big, more extensive surface area to body weight ratios and larger minute volume to weight ratios.
In addition, adolescents may be exposed to higher concentrations than adults in the exact actuation because of their shorter height, and the higher amounts of ammonia vapour or initially detected on the ground.
 Skin/eye contact
Skin/eye contact
Skin and eye irritation can occur quickly after being exposed to modest quantities of Ammonia in the air or solution. Up to a week after the following Exposure, the full extent of eye damage may not be visible. Frostbite can result from exposure to liquid Ammonia.
Ammonia gas at higher quantities can cause severe burns and injuries. Concentrated ammonia solutions such as industrial cleaners may cause corrosive harm as skin burns, irreversible eye damage or blindness if they come into direct contact with the skin.
 Frequently Asked Questions - FAQs
Frequently Asked Questions - FAQs
Questions about NH3 molecules that are frequently asked include those listed below.
 What is the name of the NH3 atom?
What is the name of the NH3 atom?
A compound of Nitrogen and hydrogen are known as NH3 is known as Ammonia. Ammonia, a colourless gas with a pungent odour, is a stable binary hydride and the simplest pnictogen hydride.
 Why is NH3 a molecular substance?
Why is NH3 a molecular substance?
A molecule is formed when three atoms of Nitrogen create a covalent bond. Even in their gaseous condition, the N-H bond and the NH3 molecule vary because they are botar. The bond N-H has a negative charge because of the difference in electronegativity between the N- and H-atoms.
 What is the reason for NH3’s pyramidal shape?
What is the reason for NH3’s pyramidal shape?
Three hydrogen atoms and an unshared pair of electrons on the nitrogen atom from the trigonometric pyramidal structure of the NH3 molecule Due to the strong hydrogen bonding between the molecules, it is a highly linked molecule.
 What is the reason for Ammonia’s pyramidal form?
What is the reason for Ammonia’s pyramidal form?
A lone pair of electrons is left after three of these pairs are used to form bonds. The tetrahedral arrangement is created when a lone pair of bonds resist each other. The ammonia molecule is trigonal pyramidal because the lone pairs are indestructible.
 Is NH3 a coordinated bond?
Is NH3 a coordinated bond?
A single hydrogen ion is exchanged from HCl to the lone pair on NH3. The electrons of this hydrogen are not transported; they remain with chlorine. As a result, the hydrogen atom’s covalent interaction with the nitrogen atom is dative.
 What causes NH3 to be tetrahedral?
What causes NH3 to be tetrahedral?
Because it contains three hydrogen atoms, the NH3 molecule has a tetrahedral geometric form. The NH3 molecular geometry has three N-H bonds. The structure remains the same after the tetrahedral linkage of the three hydrogens and one lone pair of electrons.
 Is NH3 trigonal pyramidal or tetrahedral?
Is NH3 trigonal pyramidal or tetrahedral?
The nitrogen atom in Ammonia is surrounded by four zones of the electron density (3 bonds and one lone pair). A tetrahedral arrangement is used to organize them. H-N-H angles are 106.7° in the final trigonal pyramidal molecular form.
 Why are there so many lone pairs of NH3?
Why are there so many lone pairs of NH3?
To put it another way, Nitrogen possesses five filter electrons because it is in Group 5. One electron is added to the Nitrogen’s extreme level by each of the three hydrogens, totalling electrons in four pairs. A lone pair is required because the Nitrogen is only creating three bonds.
 What is the difference between NH4 and NH3?
What is the difference between NH4 and NH3?
An ammonia gas, NH3 (also known as poisonous or free Ammonia), is a gas. The hazardous portion of Ammonia is this type. An ammonium salt, NH4 (ammonium), is safe to consume. Ammonia in ionized form. The term “total ammonia nitrogen” refers to the sum of NH3 and NH4 (TAN).
 Is NH4+ the same as NH3?
Is NH4+ the same as NH3?
The ammonium ion is NH4+. It’s positively charged with a molecular mass of 18g/mol; there is a difference between NH3-N and NH4-N in the nitrogen content of Ammonia and ammonium ions. This is the tool for those who want to monitor nitrogen levels during the treatment process.
Conclusion
This Nitrogen is half of the total nitrogen content. An effective plant nutrition balance can be achieved by using slow-release Nitrogen in the other half of the fertilizer. Once upon a time, nitric acid, hydrazine, cyanides, and amino acids were all significant ammonia products; Ammonia was a common refrigerant.
Thanks to Ammonia’s role as a key ingredient in fertilizers, our food is practically put on the table. Approximately 80% of the Ammonia produced each year is utilized to make fertilizer. Soil fertility can be maintained or even increased through fertilization.