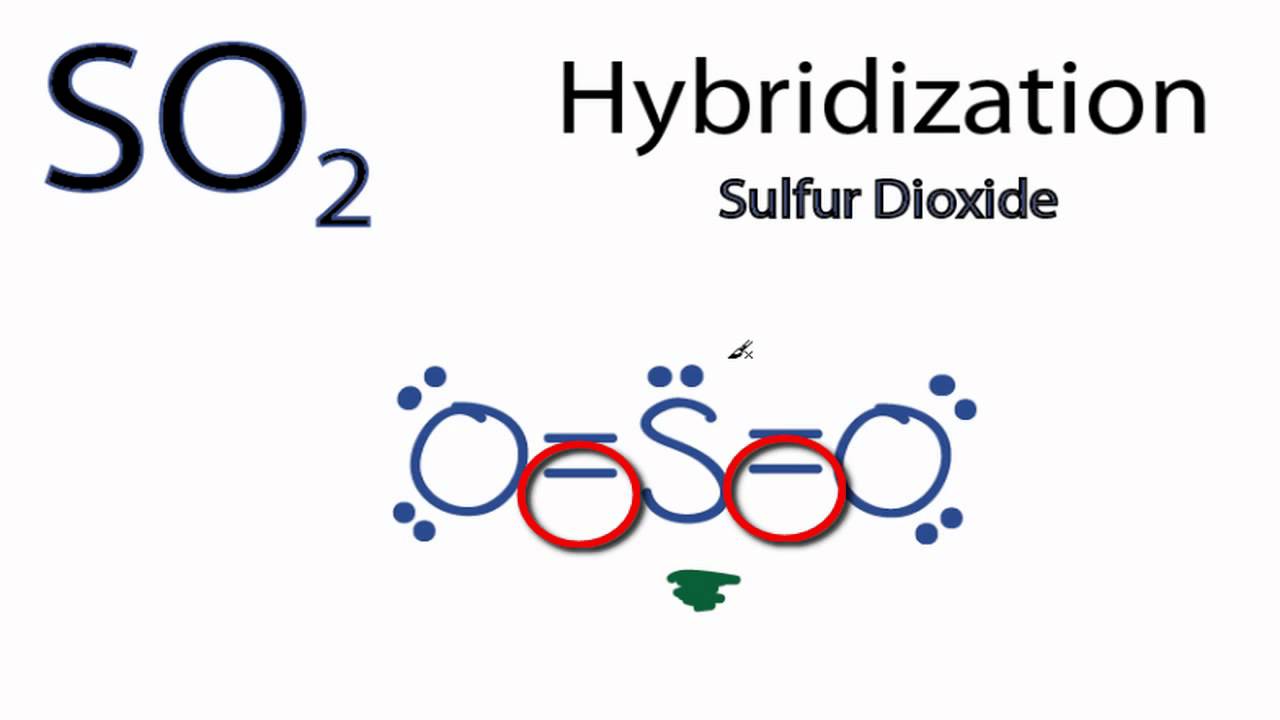
SO2 hybridization is sp2. First, we’ll examine the sulfur atom, which will serve as the center atom. O=S=O is the structure of the two oxygen atoms bound to the center atom in the production of SO2.
Hybridization of SO2
The sp2 kind of hybridization occurs in sulfur dioxide. First, we’ll look at the sulfur atom, which will serve as the center atom in our investigation.
This core atom is connected to two oxygen atoms during the production of SO2, and their structure is O=S=O. Sulfur and the two oxygen atoms are linked together by sigma and a pi connection. One single pair may be accommodated in the atom.
Let’s dissect this a little further. With six electrons in the outermost shell and no vacancies left in the innermost two, sulfide has an extremely dense ground state. 3p portal has 4 electrons, whereas 3s orbital has 2 paired electrons.
This requires four unpaired electrons to establish four bonds (with oxygen). An excited state in sulfur occurs when one of the 3px electrons in the 3d orbital jumps to the 3d orbital. One electron will be left in a 3d orbital and three will be in 3p orbitals when this occurs.
In contrast, the electrons that make up the sigma bonds and the lone pair are in distinct states of energy. When hybridization occurs, a stable state is achieved.
Two 3p orbitals and a 3s orbital combine during the hybridization process. There are three sp2 hybrid orbitals in all. In this case, two-hybrid orbitals will have unpaired electrons, while the lone pair will be in the other hybrid orbital.
As a result of these sigma bonds forming with the oxygen atoms, the electrons are left unpaired. The 3d and 3p orbitals, on the other hand, stay unchanged and are involved in the creation of pi bonds. This compound’s oxygen atom has an sp2 hybridization, which is interesting.
| Molecule’s Name | Sulfur Dioxide |
|---|---|
| Molecular Formula | SO2 |
| Hybridization | Sp2 |
| Bond Angle | 119 degree |
| Molecular Geometry | V-Shaped or Bent |
Some points to remember about SO2 Hybridization:
-
Two 3p orbitals and one 3s orbital hybridize in SO2 hybridization.
-
Unpaired electrons are found in two-hybrid orbitals, whereas the lone pair is found in one hybrid orbital.
-
They form pi bonds because 3d and 3p orbitals are identical.
Outline:
Here’s a short tip: When one S orbital joins two p orbitals, the outcome is a hybridization called Sp2 that has three equivalent orbitals. Similarly, the electrical arrangement for SO2’s ground state is 1s2 2s2 2p6 3s2 3p4. One electron from the 3px orbital transfers to the 3d orbital when the atom is excited. Consequently, we have 3p3. Sp2 hybridization is formed using three analogous orbitals, each of which contains two pairs and two unpaired electrons.
How to Prepare SO2 Chemically?
There are a variety of ways to create SO2. For your convenience, I’ve broken down each technique into its parts.
-
The contact process is the primary source of SO2 generation during the synthesis of sulfuric acid. This is the most common technique for producing SO2 in industrial settings.
-
There are many historical connections between chemistry and history; here’s one for you! For sulfuric acid production, the United States utilized 23.6 million tonnes of SO2 in 1979.
-
Burning sulfur or sulfur-containing compounds produces SO2.
-
S + O2 ——-> SO2
-
2 H2S + 3O2 ——–> H2O+2SO2 are the formula.
-
The roasting of pyrite, sphalerite, and cinnabar may also be used to produce SO2 ( sulfide ores).
-
A byproduct of calcium silicate cement production is the production of SO2.
-
In this case, 2 CaSO4 + 2SiO2 + C ————> 2CaSiO3 + 2SO2 + CO2
-
Copper turnings and heated concentrated sulfuric acid react in the laboratory to produce SO2 as a byproduct.
-
When Cu is added to 2H2SO4, the reaction proceeds as follows: CuSO4 + SO2, + 2H2O.
-
A substantial volume of SO2 may be produced by natural disasters, such as volcanic eruptions.
SO2 Lewis Structure
First, let’s have a brief explanation about the significance of lewis’s structure and the procedures involved in drawing it before moving on to SO2.
A compound’s Lewis structure is the arrangement of electrons around its atoms. This diagram reveals the kind and number of bonds that make up the compound.
In the next section, we’ll explain how to draw a lewis structure:
-
The first and most critical step is to determine the total amount of valence electrons in the molecule. Take care of the + and – signs while you’re at it. A “+” sign indicates a loss of electrons, while a “-” sign indicates again.
-
It’s now time to identify the core atom. The center atom has the greatest number of binding sites.
-
Single-bond structures are used in the third stage to build a skeleton.
-
After the single bonds have been formed, we must complete the octet of atoms by adding the remaining electrons. Electropositive atoms should always come after the electronegative ones.
-
To satisfy the octet rule for all atoms, it is required to provide double or triple bonds.
-
Finally, make sure that all the atoms are at their lowest possible formal charge before moving on.
Let’s have a look at SO2’s Lewis structure now.
-
The valence electron of sulfur in SO2 is 6
-
There are 6 valence electrons in oxygen
-
Because the complex contains two oxygen atoms, it is equal to 6*2 = 12 atoms.
-
Valence electrons in all = 18
No single bond can be found on any atoms once we’ve drawn the skeleton structure. That’s why a second connection is necessary. As a result, double bonds have an electron count of 8.
We’re left with 10 electrons left after subtracting them from the total valence electrons. The leftover electrons must be distributed around the atoms by the specification. This will bring the atoms’ octet to a close. Both sulfur and oxygen have one lone pair.
Summary:
So, if we wish to describe the Lewis structure of SO2, we can say that there are two double bonds between the sulfur and oxygen atoms in the molecule, as seen in the diagram below. In addition, the sulfur atom has a lone pair, and each oxygen atom contains two lone pairs in the SO2 Lewis structure.
Sigma Bonding of Sulfur & Oxygen
Oxygen’s 2s and 2p orbitals combine to form hybridized sp2 AOs for bonding (the hybridized orbitals are represented by the “O sp2” label on the far right of the picture), which have a power level in between the two distinct 2s and 2pAOs.
Hydrogen bonds are formed by combining electrons from the three sulfur atoms’ three sp2 orbitals (the “S sp2” label on the figure indicates this) to form hybridized sp2 orbitals (the “S sp2” label indicates this by way of the “S” label on the diagram).
Sulfur and oxygen may overlap to produce one bonding and one *antibonding MO when their sp2 AOs (2s+2px+2py) are near one other.
The bonding MOs are numbered 1 and 2 for the left and right SO bonds, respectively. Empty antibonding MOs may be seen in this image.
In the end, we’ll notice that we’ve taken into account that every oxygen has a sulfur atom bonded to it. Four is the number of electrons we have so far.
Pi Bonding of Sulfur & Oxygen
Finally, let’s have a look at the ties. Each sulfur-oxygen link has an extra bond that needs two more electrons.
The 2pz AOs of each oxygen and the 3pz of sulfur form bonds, according to the summary. These orbitals may overlap side-by-side to create bonds since the z-axis, which is moving away from us, was described as lying along the z-axis, and we hadn’t taken into account the one’s orbitals, which are located along the z-axis as well.
Similarities & Dissimilarities B/W Sulphur & Oxygen
| Similarities | Dissimilarities |
|---|---|
| The outer electric configuration of both O and S is ns2 and np4. | Sulfur, on the other hand, has three allotropic forms, while oxygen only has two. |
| In the presence of metals, the oxidation state of both reacts to be -2. | Both Oxygen and Sulphur are combustible, hence they aid in the combustion process. |
| Normally, O and S are divalent metals. | Sulfur is a solid at room temperature, while oxygen is a gas. |
| Metals and nonmetals may create covalent compounds, such as water, sulfur dioxide, carbon dioxide, and CS2. | While oxygen is paramagnetic, sulfur is diamagnetic. |
| Only non-metals O and S may be found in this set. | In contrast, Sulphur is insoluble in water, whereas oxygen is readily soluble in water. |
| Each one has a distinct allopatric shape. | When steam is pushed over boiling sulfur, minimal hydrogen sulfide and sulfur dioxide are produced, but oxygen does not react with the water. |
Frequently Asked Questions
Here are some FAQs related to Hybridization:
1. What is the reason behind SO2’s sp2 hybridization?
It is possible to have up to three sp2 hybrid orbitals in your atom. One hybrid orbital will contain the lone pair of electrons, whereas the two-hybrid orbitals will have unpaired electrons. Unpaired electrons will then form sigma bonds with oxygen atoms as a result of this. It should be noted that the oxygen atom in this molecule has been hybridized through sp2 sp3.
2. Is sp2 present in SO2?
The sigma and pi bonds in SO2 hybridization are discussed in detail. The core sulfur atom has an sp2 SO2 hybridization. Each of the oxygen atoms is also sp2.
3. So, what is this thing called “SO2 shape?”
Because it comprises two oxygen atoms twisted in a V shape and two corners with one lone pair of electrons on the center sulfur atom, the SO2 molecule has a V-shaped or bent geometry. The SO2 molecular geometry has two S-O double bonds.
4. In what way are SO2 and SO3 intertwined?
For the sulfur atom in the middle, the SO2 hybridization is sp2. Each of the oxygen atoms is also sp2. SO3 hybridization, involving the and sigma bonds, is described. For the carbon atom in the center, the SO3 hybridization yields sp.
5. Why are there lone pairs in SO2?
In all, there are 18 valence electrons in sulfur dioxide: 6 are provided by the sulfur atom and 6 are provided by the two oxygen atoms. The sulfur atom has the remaining two valence electrons put on it as a single pair.
6. Is SO2 trigonally planar or trigonally bent?
For example, the electron-domain geometry of sulfur dioxide (SO2) is trigonal planar. Sulfur contains six valence electrons, but only one non-bonding lone pair. These six valence electrons form two single bonds with two oxygen atoms. There are three distinct electron domains in sulfur, explaining this phenomenon.
7. Is SO2 a linear gas?
The SO2 molecule has two pairs of binding sites and a single electron pair. However, in contrast to CO2, which lacks a single electron pair and so has linear geometry, because of the bond angle reduction caused by this attraction, the bond angle of the OH-molecule drops from 120° to 119.5°.
8. What is the relationship between sp2 and sp3 hybridization, and why is it important?
One S and one p atomic orbital combine to form sp hybridization, two S, and two p atomic orbitals mix to form sp2 hybridization, and three S and three p atomic orbitals mix to form sp3 hybridization.
9. What is the C’s hybridization in C2F2?
C2F2, or Difluoroacetylene, is a great chemical to play with. The steric number is needed to determine the atom’s hybridization. For each atom, just sum up the number of electron pairs and the number of protons and neutrons it is bound to (double and triple bonds count as one). The Carbons have an sp hybridization and are bound to two atoms apiece.
10. In s02, how many pairs of lone individuals may be found?
In contrast to the sulfur, which has just one remaining lone pair of electrons, each oxygen atom possesses two. As a result, each of SO2’s atoms has five lone pairs on its final shell.
Conclusion
This page covers almost every aspect of SO2 that you could ever want to know. Before you start studying the reactions and equations involving SO2, read this page to make sure you understand all you need to know about Lewis’s structure, geometry, hybridization, and the MO diagram of SO2.