Methane Lewis structure contains four C-H bonds. The valence shells of carbon atoms do not contain any lone pair. The methane lewis structure is easy to draw because carbon is the central atom in methane molecule.
Lewis Structure of CH4
As the carbon-hydrogen atom lewis structure indicates, it takes eight valence electrons to complete the shared bonding required of a single CH4 molecule.
Step-by-step instructions on drawing the Lewis dot structure for the CH4 molecule are provided here.
![]() A single CH4 molecule requires sixteen total valence electrons, so this is the first step.
A single CH4 molecule requires sixteen total valence electrons, so this is the first step.
![]() A single CH4 molecule must then search for electrons in order to achieve a stable state.
A single CH4 molecule must then search for electrons in order to achieve a stable state.
![]() For a single CH4 molecule, four carbon atoms and one hydrogen atom each are required, so the total number is eight.
For a single CH4 molecule, four carbon atoms and one hydrogen atom each are required, so the total number is eight.
![]() A CH4 molecule contains a total number and type of bond-forming atoms.
A CH4 molecule contains a total number and type of bond-forming atoms.
![]() Each carbon and hydrogen atom has a single shared covalent bond formed between them (C-H).
Each carbon and hydrogen atom has a single shared covalent bond formed between them (C-H).
![]() Finally, look for the molecule’s single central atom. In the case of methane, it is carbon (CH4).
Finally, look for the molecule’s single central atom. In the case of methane, it is carbon (CH4).
Valence Electrons of Methane
The electrons in an atom’s outermost shell, known as the valence shell, participate in bond formation. By either giving or receiving an electron from another atom, these electrons help form bonds between them.
An atom’s valence electrons can have a maximum of eight. The atomic number of carbons, which is six, is necessary in order to determine the atom’s valence electron count. As a result, the carbon will have an electronic configuration of 1s2 2s2 2p2.
The p shell has a shortage of four electrons because it needs to hold six electrons. This has resulted in the carbon atom’s having four valence electrons. Its electronic configuration is 1s1 because it has the same atomic number as hydrogen.
Due to a shortage of one electron, a hydrogen atom has only one valence electron.
|Bonded atoms|Lone pair|Generic formula|Hybridization|Molecular geometry|Electron geometry|
|----|----|----|----|----|----|----|
|1|0|AX|S|Linear|Linear|
|2|0|AX2|Sp|Linear|Linear|
|1|1|AXN|Sp|Linear|Linear|
|3|0|AX3|Sp"|Trigonal planar|Trigonal planar|
|2|1|AX2N|Sp’|Bent|Trigonalplanar|
|1|2|AXN2|Sp|Linear| Trigonal planar|
|4|0|AX4|Sp’|Tetrahedral|Tetrahedral|
|3|1|AX3N|Sp’|Trigonal pyramid|Tetrahedral|
|2|2|AX2N2|Sp’|Bent|Tetrahedral|
|1|3|AXN3|Sp’|Linear|Tetrahedral|
|3|2|AX,N,|Sp"d|T-shaped|Trigonal bipyramidal|
Summary
It is the outermost electrons, known as the valence electrons, of an atom that are involved in the formation of bonds. A maximum of eight valence electrons can be found in an atom. The valence electron count of carbon must be determined using its atomic number, which is six.
Octet Rule
According to this rule, an atom can only have eight valence electrons drawn around it. Carbon has four valence electrons in short supply, whereas hydrogen needs only one valence electron function.
To satisfy the atoms’ need for valence electrons, the Lewis structure of CH4 is drawn.
CH4 Bond Angles
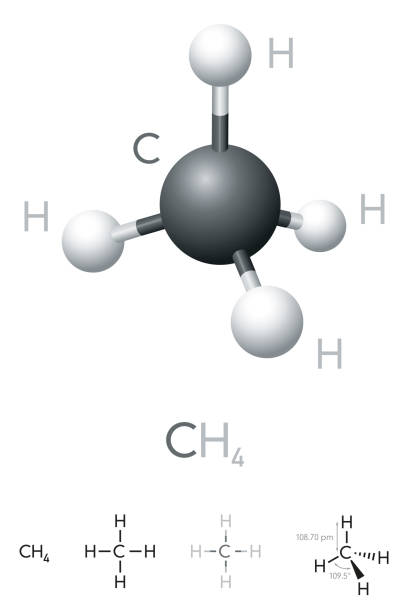
Molecular geometry and bond angles for any molecule can be determined using AXN Notation. According to the table below, bond angles in CH4 are 109.5°, because CH4 follows the AX4 notation here.
Because of its spherical shape, the CH4 molecule has a 109.5° of bond angle. Because of the distortion caused by the lone pairs in a molecule, the bond angles of that molecule change. Because there are no lone pairs of electrons in this molecule, the bond angle of H–C–H is 109.5 degrees.
The Geometrical Structure of Methane (CH4)
No lone pairs can be found on any atom of methane (CH4) in the tetrahedron. The Valence Shell Electron Pair Repulsion (VSEPR) theory is used to explain this behaviour. Using a computer model, this theory is used to predict the shape of a molecule and the reason for this shape.
This theory asserts that as there is no distortion in the CH4 molecule’s structure, it is an ideal bent-shaped molecule or tetrahedron with a bond angle of 109.5 degrees between hydrogen, carbon, and hydrogen (H-C-H).
Methane is a nonpolar molecule because of the symmetrical shape of the bonds formed in the CH4 molecule; the charges on its atoms are evenly distributed. You can learn more about the polarity of CH4 in the article written on the subject.
There are lone pairs and bond lengths that distort bond angles in molecules, which is why the ideal bond angle isn’t achieved. It is clear from the Lewis structure that the carbon atom and the four hydrogen atoms share an equal number of electrons.
Because of this, methane’s structure is extremely stable in the natural world.
In short
Since its atoms are symmetrical in shape, methane is nonpolar. It is possible to foretell a molecule’s shape by employing the [Valence Shell Electron Pair Repulsion Theory (VSEPR). According to this theory, the structure of the CH4 molecule is unaltered.
Hybridization in Methane (CH4)
When two atomic orbitals are mixed together and overlapped, the result is a completely new set of orbitals with the same energy, known as new hybrid orbitals. In methane (CH4), the carbon atom hybridization is found to be sp3.
Carbon’s one 2s and three 2p orbitals mix and overlap to form four new hybrid orbitals with equal energy and a similar shape, and this is the reason for this. In addition, the new four sp3 hybrid orbitals have 25% of the s orbital characteristics and 75% of the p orbital characteristics.
Carbon-hydrogen (C-H) sigma bonds are also formed using these four new hybrid orbitals created by the four hydrogen atoms. The single shared covalent bond contains only one sigma bond and no pi () bonds.
As a result, a methane molecule lacking a pi bond forms four sigma bonds, each of which contributes to the carbon atom’s hybridization.
Summarize
In methane (CH4), the carbon atom hybridization is found to be sp3. Carbon-hydrogen (C-H) sigma bonds are also formed using these four new hybrid orbitals. The single shared covalent bond contains only one sigma bond and no pi bonds.
Molecular Orbital diagram of CH4
Hybridization type can be determined by looking at the molecular orbital diagram, which shows how mixing and overlapping have taken place. Carbon’s four sp3 hybrid orbitals are shown in the figure to mix and overlap with hydrogen’s four 1s atomic orbitals.
The only occupied sp3 hybrid orbital of the carbon overlaps head-on with the 1s orbital of the hydrogen in each carbon-hydrogen bond (C-H). It can be confirmed by the fact that only sigma bonds overlap head-on, whereas pi bonds overlap lateral.
Because there are no pi bonds in the CH4 molecule, only head-on overlapping occurs. As evidenced by the apparent phase shift between the carbon and hydrogen atoms shown in the diagram, all four of the top orbitals are empty.
These orbitals can only be filled with electrons if the bonding energy between the carbon and hydrogen atoms is reduced. The non-bonding energy level fills all four of the bottom orbitals, but the energy levels at the top are empty.
The lowest-energy molecular orbital is evenly dispersed throughout the molecule.
To summarize
Carbon and hydrogen atoms are the building blocks of the CH4 molecule. The four sp3 hybrid orbitals of carbon and hydrogen mix and overlap. Because there are no pi bonds, only sigma bonds can overlap head-on.
Methane polarity: is CH4 polar or nonpolar
We know that the dipole moment of a polar molecule is caused by the unequal distribution of charges, whereas the dipole moment of a non-polar molecule is cancelled out by the symmetry of the molecule’s shape.
Is methane polar or non-polar? There are four bonds in CH4 that are symmetrically arranged into tetrahedral geometrical shapes, making it a non-polar molecule because of this arrangement. Since the dipole moment generated on each side of C-H will cancel out each other, the molecule will be nonpolar in the overall sense.
Three factors that indicate the polarity of CH4
1. Electronegativity:
To understand how polarity is formed, one must understand how electronegativity is related to the difference in electronegativity between two or more atoms or molecules. Electronegativity refers to an atom’s propensity to draw electrons towards itself.
The polarity will be higher if the electronegativity difference between the atoms is large. Take a look at carbon’s and hydrogen’s electronegativity now.
Compared to hydrogen’s electronegativity of 2.2, carbon’s is about 2.6. To put it simply, carbon has a greater capacity for attracting and retaining electrons.
As a result, the Pauling scale states that if the electronegativity difference between two atoms is less than half of one, the bond is nonpolar. Carbon and hydrogen have an electronegativity difference of less than half.
This means that all dipoles generated along CH4’s C-H bonds cancel each other out, resulting in a nonpolar molecule because CH4’s symmetric tetrahedral geometry cancels them out.
2. Dipole moment
The carbon-hydrogen dipole moment ensures the polarity’s strength. This is because the greater the molecular dipole moment is, the more strongly it polarises the molecule. There were positive and negative charges induced by the difference in electronegativity between carbon and hydrogen.
Consequently, the carbon atom has a slightly negative charge and the hydrogen atoms have a slightly positive charge because carbon is more electronegative and hydrogen is less so. As a result of the C–H bond and these charges, there are four dipole moments.
Carbon is more electronegative than hydrogen, which causes a dipole moment in the direction from hydrogen to carbon. However, because CH4 has a regular tetrahedral structure with no lone pair on the central atom, dipole moments generated along these bonds can easily be cancelled out.
The dipole moment can be expressed mathematically as D = Q*R.
The charge on the atoms multiplied by the distance between them is the formula for the dipole moment.
3. Geometrical or molecular shape
The polarity nature of a molecule is strongly influenced by its geometrical structure. Its tetrahedral structure has four nonpolar covalent bonds (C-H), but all of them are in the opposite direction of the molecule’s molecular structure. There are no lone pairs in the centre of CH4, so it has an extremely symmetrical structure.
To make it nonpolar, all of the C-H bonds are in the exact opposite direction, making them highly symmetric and easy to cancel out.
Sum up
The tendency of an atom to attract electrons to itself is known as electronegativity. An object’s geometrical structure greatly affects its ability to attract or repel electrons. This is due to the fact that a stronger polarisation of the molecule occurs with an increased molecular dipole moment. The dipole moments of carbon and hydrogen determine their polarity.
Frequently Asked Questions
Following are some frequently asked questions related to the methane Lewis structure.
1. Why is the molecular geometry of CH4 is same as its electron geometry
The VSEPR theory states that the molecular shape of CH4 is tetrahedral, but that the tetrahedral electron geometry is also tetrahedral because the electron geometry of CH4 is the same as the molecular shape.
According to the Lewis structure, a lone pair does not exist on CH4’s central carbon atom. Since CH4’s geometry is determined only by the atoms that are bonded together.
Because of CH4’s Molecular geometry = CH4’s Electron geometry.
2. What is the geometry and shape of CH4?
For CH4, the bond angle HCH = 109.5° defines the tetrahedral molecular geometry or shape. There are no lone electron pairs in the central region of CH4, so the electron geometry is tetrahedral.
3. Is CH4 tetrahedral?
Methane is tetrahedral, with four equal bond angles of 109.5°, four equal bond lengths, and no dipole moment.
4. What type of bond is CH4?
It is a covalent compound with exactly five atoms linked by covalent bonds: methane, CH4. A Lewis structure represents this type of covalent bonding. The covalent bonds are represented by the lines or sticks, as the case may be. Four hydrogen atoms (H) are linked to a central carbon (C) by four bonds (H).
5. What polarity is CH4?
Composed of one carbon atom and four hydrogen atoms, methane (CH4) is a non-polar hydrocarbon. Carbon and hydrogen’s electronegativities are not great enough to form polarised chemical bonds with methane, which is why it is non-polar.
6. What is the octet rule of CH4?
To us, carbon is a key component of all organic molecules because it is the building block of all of them. With four electrons in its valence shell, carbon will need four more electrons to meet the octet rule. Because of this, it must be combined with four hydrogen atoms to form methane, a stable compound (CH4).
7. Is CH4 a pyramid?
Ammonia, NH3, is a pyramid-shaped molecule with an H-N-H angle of 107 degrees and hydrogens arranged in an equilateral triangle. The H-C-H angles in a CH4 tetrahedron are all equal to 109.5°.
8. Why does CH4 have a tetrahedral shape?
In the case of methane, all four valence orbitals are bonding orbitals, which are arranged around the carbon in the kind of tetrahedral geometry that minimizes electronic interaction.
9. What is the electronegativity and chemical bond of CH4?
Carbohydrates have an electronegativity of 2.55, whereas hydrogen has a value of 2.20. There is a difference of 0.35 between the two. The difference must be between 0.5 and 1.5 to qualify as “polar covalent” bonds.
10. Why is CH4 not square planar?
The carbon atom in a CH4 molecule is sp3 hybridised, giving it a tetrahedral structure. In order for CH4 to be square planar, it needs dsp2 hybridization, which is not possible in carbon due to the absence of the d-orbitals.
Conclusion
Methane (CH4) has four single shared covalent bonds between each of the carbon and hydrogen atoms in its Lewis structure. Since only sigma bonds are present, the hybridization of CH4 is sp3 due to its one 2s orbital and three 2p orbitals, producing four new hybrid orbitals.
Even though the new hybrid orbitals have sigma bonds, they still have many of the characteristics of a p orbital. As a result of having no lone electron pairs on an atom, the bond angle is an ideal 109.5° (the tetrahedral angle).
Related Articles
What Is The Molecular Geometry Of Pbr3
Nh3 lewis structure molecular
N2O Molecular Geometry