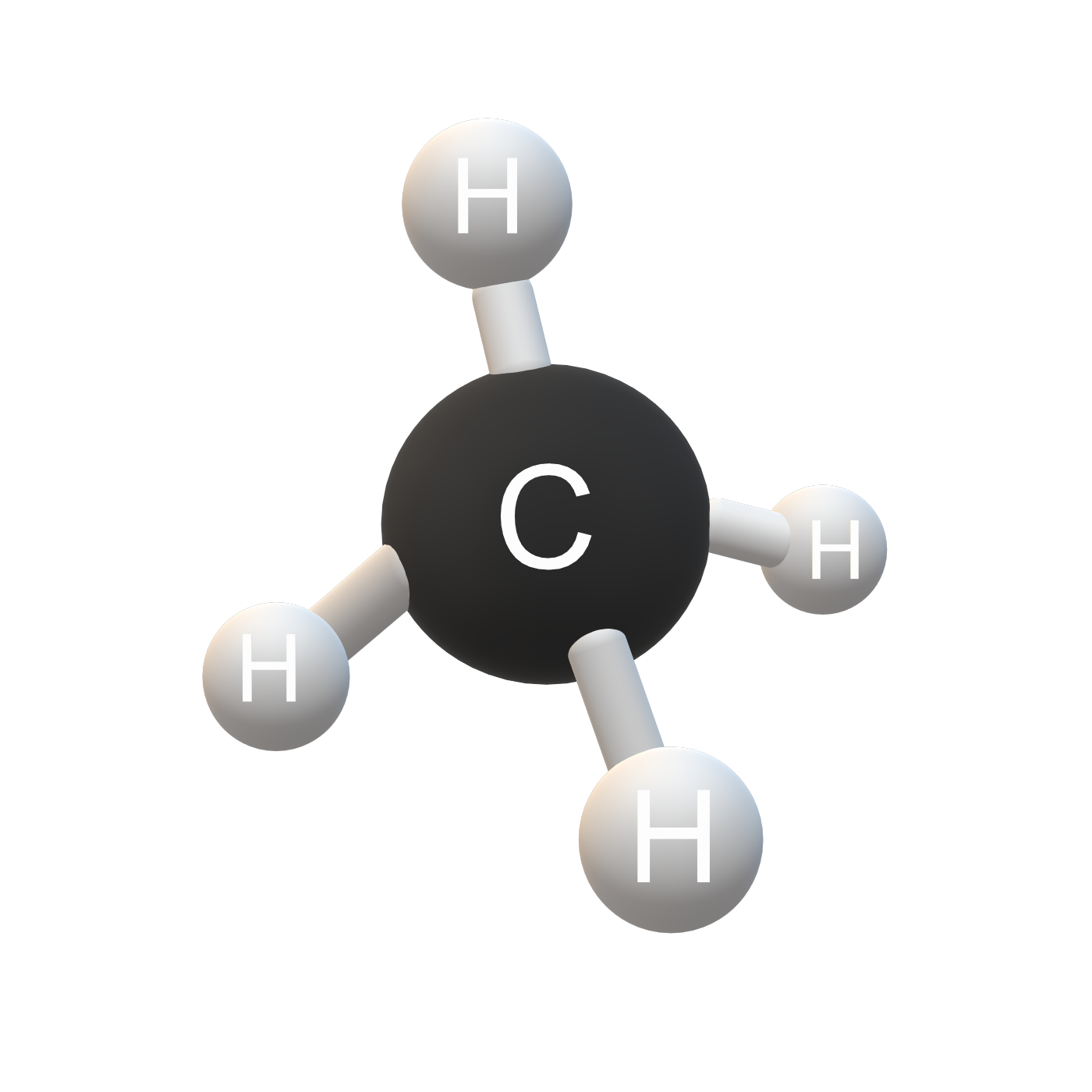
CH4 molecular geometry: CH4 molecular geometry is tetrahedral and the electron geometry of CH4 is also tetrahedral. CH4 is the chemical formula of methane which is a gas that is odorless and colorless in nature. Chemically it is formed by one atom of carbon and two atoms of hydrogen.
What is CH4(methane)
CH4 is the chemical formula of methane which is a gas that is odorless and colorless in nature. Chemically it is formed by one atom of carbon and two atoms of hydrogen. Methane is a hydrocarbon of the simplest form and is mainly used for generating electricity and as a fuel in gas turbines and steam engines.
This article will cover all the concepts of methane and CH4 molecular geometry, Lewis dot structure, its properties, and all the other important information about the colorless gas which is CH4. Methane is usually used for industrial purposes and as a fuel product for many things like steam engines, turbines, etc. Methane is also used for preparing other chemicals.
CH4(methane) explained through chart
|||
|—|—|—|
| Molecule name| Methane|
| Molecular geometry and shape of methane CH4 |Tetrahedral|
| CH4 hybridization |Sp³|
| Bond angle of CH4 |109.5°|
|Total value of valence electron for CH4|8|
| Formal charge |0|
Uses of CH4(methane)
Methane is used for the production of so many important chemicals which become a huge part of industrial products. Methane is one of the biggest sources of producing hydrogen and it produces hydrogen and carbon monoxide after reacting with steam at high temperatures. The by-products of this reaction are used for the production of Ammonia for making explosives and also used in fertilizers.
Some very important chemicals are derived from methane are chloroform, nitromethane, Carbon tetrachloride, and methanol. Carbon black is one of the very important products as a result of methane which is used in the manufacture of rubber used for the making of tires for automobiles.
-
Methane is widely used in the manufacturing of automobiles as it plays a huge role in the making of rubber used for tires.
-
Water heaters and ovens are also run by methane being the fuel. These types of heaters and ovens require gases that are in higher concentrations and can be abundantly manufactured on a large scale.
-
Main use of methane is generating electricity which is a very important reason in modern society. Without Methane the electrical power will go down and it is very hard to find an alternative to methane.
-
Methane in its refined liquid form is used as fuel for rockets.
-
Methane has a very huge use in industrial products and it is used as an antifreeze Ingredient for industries.
-
It is commonly used as an ingredient in fertilizers.
-
It is also widely used to sanitize products.
-
Methane is very useful in the testing process of gas Appliances.
-
It is used in gas cookers and gas-fired power stations.
Chemical properties Of CH4(methane)
Methane is a colorless and odorless gas that is lighter than air in nature. The chemical formula for methane is CH4. Methane gas highly reacts in the air by forming carbon dioxide and water vapor as a byproduct. It is a hydrocarbon and a part of the simplest paraffin series.
Methane gas burned in the air creating a pale flame. this gas is formed abundantly in the atmosphere and is a byproduct of certain human activities. The flame created by methane is very hot and luminous. The gas is neutral and is slightly soluble in water but a specific concentration of water and methane mixture can cause explosions.
Methane being the cause of explosions is commonly found in collieries and coal mines specifically. This kind of eruption can cause massive disaster and this kind of mixture should be avoided for safety.
Production of CH4(methane)
Methane is mainly produced by the natural processes which happens underwater usually. The bacterial decomposition of vegetables underwater is an anaerobic process that produces methane. This is why it is sometimes called marsh gas or swamp gas. The wetlands have proven to be the main source of methane production.
Some other sources where methane is found and produced are by the digestive system of termites, a by-product of volcano ruptures and ocean vents. Methane hydrate is also a source of production through industrial processes and it can also occur in arctic ice. Also, there is 50 to 90% of methane present in natural gas which results in the use of burning coal and is used as a flammable gas for industrial purposes.
When flammable products are going through the combustion process the production of natural gas is the main source of human activities based production of methane. Industrial processes that include the usage of Decomposing and decaying waste becomes a human-based source of methane gas. The distillation and coke oven processes are also the sources of methane gas production.

CH4 Lewis structure
Lewis dot structure of methane CH4 is very simple to draw because it has carbon as the central atom while the four hydrogen atoms spread around the central atom. The molecule of methane does not have any Lone pairs of electrons. Here is the guide and stepwise process for drawing Lewis’s structure of methane CH4.
1. Step one
The very first step you need to take for drawing the Lewis structure of methane is that you have to find out the number of valence electrons present in the central and terminal atoms of a molecule. The reason behind this process is so that we can use that calculated amount of ions to distribute them among the Central and terminal atoms to complete the shell.
There are two basic ways of finding out the valence electron of a particular atom which is by checking out the periodic group or by writing out their electronic configuration. Finding out the valence electron value through the periodic table is much easier so we will use this method in our further steps.
So as we know that atom carbon belongs to group 14A or 4A which gives carbon 4 valence electrons on its outermost shell. While hydrogen is from group 1A of the periodic table then it has one valence electron in its outer shell. So the number of valence electrons available for the drawing of Lewis structure is:
Carbon valence electron = 4
Hydrogen valence electron= 1 (1×4)
Therefore, 4+4 = 8
CH4 has a total value of valence electron of 8
Now that we have found out the value of valence electron in methane
molecule now let’s move forward to the second step.
2. Step two
The second step is to find out the least electronegative atom and put it in the center position of a molecule. Now, this particular step is easier when the molecule has hydrogen in it, because whenever a molecule has hydrogen in it and you have to draw the Lewis structure then it is always placed at the outer side of the structure.
To complete its outer shell, hydrogen only needs two electrons. So it does not matter which atom has more electronegativity when hydrogen is present in a molecule. In that way the carbon atom will be placed in the center of the diagram and all the hydrogen atoms will be spread out in the outer part of the diagram.
3. Step three
The third step for the Lewis structure is to connect the outer atoms to the central atom through a single bond. A single bond that is used to connect the two atoms carries 2 electrons. So as you can see in CH4 molecular geometry, The structure contains four hydrogen atoms and one carbon atom.
Carbon works as the central atom while the four hydrogen atoms are connected with a single bond with the value of two electrons each. In the first step, we found out the value of the valence electron of this molecule and the value was eight.
Therefore, Methane has four single bonds connecting the central and terminal atoms which means all the 8 valence electrons are used in the bonding of all the atoms in the CH4 molecule.
Now we need to find out if carbon and hydrogen have completed their octet shell. Carbon needs 8 electrons to complete its shell and as we can see that each bond carries 2 electrons and carbon is connected with four bonds in the molecule which means carbon completed its octet shell.
Hydrogen needs two electrons to complete its shell and each hydrogen atom has a single bond connected to it which gives two electrons meaning hydrogen also completed its octet shell.
CH4 electron geometry and molecular geometry
The molecular geometry of Methane Ch4 is tetrahedral and the carbon central atom shares a single covalent bond with all four atoms of hydrogen. This molecule has no lone pairs of electrons and all the electrons around the central atom will repulse and try to create distance from each other and take position around the central atom where it can be comfortably placed.
One of the reasons behind the tetrahedral structure of methane CH4 is that this molecule has no lone pairs of electrons and the electron pairs in the outer structure repel each other and take positions as far away from each other and form a stable structure.
In this position the tetrahedral formation of CH4 is complete and all the hydrogen atoms are spread on the corners of the structure forming a bond angle of 109.5°.
CH4 molecular geometry and polarity
So we know that a dipole moment is created when both of the atoms in a molecule have unevenly distributed electrons across the molecule. This type of dipole moment only occurs in polar bonds, nonpolar bonds do not cause the dipole moment and hence they have evenly distributed charges in their molecule.
Nonpolar molecules or bonds create a symmetrical angle or shape which prevents the dipole moment and both sides canceling out each other.
The question is whether methane CH4 is polar or nonpolar? Methane is a nonpolar molecule because it has four covalent bonds between carbon and hydrogen which make the distribution of charges even and the dipole moment cancel each other out because of the symmetrical shape of the molecule.
The atoms are symmetrically arranged in a tetrahedral structure in methane CH4 which makes it completely a nonpolar molecule.
Factors indicating the polarity of methane CH4
-
Electronegativity plays a huge role in indicating the polarity of a molecule as the electronegativity difference between atoms and molecules is directly proportional to the polarity of a molecule. By finding out the differences between the EN values of all the atoms in a molecule we can determine whether the molecule is polar or nonpolar. The electronegativity of an atom is responsible for attracting the electrons do itself. The higher the rate of electronegativity difference between the atoms, the more polar will be a molecule. Therefore if we check out the electronegativity of carbon and hydrogen in the molecule of methane. The EN value of carbon is around 2.6 and of hydrogen is 2.2. In this case, carbon has a higher tendency of attracting electrons toward itself. As you can calculate that the electronegativity difference between carbon and hydrogen in methane is less than 0.5 which makes it a nonpolar bond according to The Pauling scale.
-
Dipole moment is responsible for strengthening the polarity between the atoms. The greater the dipole moment is present between the atoms the more polar a molecule is going to be. The dipole moment in a molecule between atoms induces the distribution of positive and negative charges. Now carbon being more electronegative than hydrogen creates a negative charge and the hydrogen atom is left with a partial positive charge. Along with C_H bonds, these charges create 4 dipole moments. Even though the atoms in the CH4 molecule create 4 dipole moments, the tetrahedral structure of methane is symmetrical and it cancels out the dipole moment. Leaving it with zero lone pair on the central atom.
-
The molecular or geometrical shape of a molecule can play a vital role in determining its polarity. Methane CH4 molecular geometry is tetrahedral with four covalent bonds all in different directions. The bonds between carbon and hydrogen in the molecule of methane are widely separated from each other which gives it a tetrahedral and symmetrical structure. And when a molecule has a symmetric structure then it means it is nonpolar. Methane CH4 does not contain any lone pairs in the central atom and it has single covalent bonds between carbon and hydrogen. The opposite directions of all the atoms cancel out the dipole moment and hence the molecule of methane becomes nonpolar.
CH4 hybridization
When we talk about understanding the hybridization of methane, we need to find out about the different shapes of their atomic orbitals. There are also different types of hybridization processes depending on the energy levels of the molecule. The molecule of methane involves SP³ hybridization. As we know that CH4 is a combination of two elements which are 1 carbon and 4 hydrogens atoms.
Carbon and hydrogen complete their octets by bonding to each other. Carbon takes 4 valence electrons from the hydrogen octet while hydrogen also completes its octet shell through the central atom. After the completion of their octet shells, Covalent bonds are formed.
The central atom carbon of methane molecule is SP3 hybridized because one 2s orbital and three 2p orbitals in carbon valence shells of the same shape and energy combine to form sp3 orbitals. Later on, hydrogen uses the same SP3 hybridization orbitals to create C_H sigma bonds with carbon to form the molecule of methane.
Important points for methane hybridization
-
Every SP3 hybrid orbital of carbon overlaps the 1S orbital of hydrogen to create C_H sigma bonds in methane.
-
There are no lone pairs while the hybridization involves the mixture of 1 S orbitals and 3 p orbitals.
-
The SP3 hybrid orbitals contain an unpaired electron each. And these orbitals are of equal shape and energy.
Frequently asked questions
Some related questions are answered below:
1. Why does CH4 have a tetrahedral shape?
Methane CH4 uses 8 electrons to complete its octet shells and to form bonds between carbon and hydrogen atoms. To avoid the repulsion happening in the outer side of the molecule, the atoms tend to spread on the outer corners and away from each other which forms a tetrahedral structure with an angle of 109.5°.
2. Does CH4 have polar bonds?
The polarity of the molecule occurs when there is an uneven distribution of Valence electrons. But in methane CH4 the valence electrons are equally shared among the atoms in a molecule so it is completely nonpolar and there is no net value of polarity in CH4.
3. How many bonding atoms are in CH4?
Methane has four bonded groups and there are no lone pairs found in the molecule.
4. Why is CH4 not polar?
If the molecular geometry of a molecule tends to be symmetrical then all the dipole moments cancel out each other. CH4 is not polar because all the hydrogen atoms that surround the carbon atom have similar dipole moments and go in the same direction towards the central atom carbon. therefore CH4 is not polar and completely shows properties of nonpolar bonds.
5. Does CH4 have all equal bond angles?
CH4 has a tetrahedral structure so it is supposed to have 4 bonds with an angle of 109.5°. All four bonds are equal with equal length as well. And there is no dipole moment in methane.
6. How many atoms are in CH4?
Methane whose chemical formula is CH4 has a total of five atoms in its molecule which is: One central atom of carbon and four atoms of hydrogen.
7. How many valence electrons does CH4 have?
CH4 has a total valence electrons of eight which makes it a covalent bond and forms four pairs. CH4 has eight electrons and pairs of bonds are available in its module.
8. Is CH4 a molecule or atom?
The elements present in CH4 are carbon and hydrogen, both are nonmetal elements and the formation of covalent bonds between hydrogen and carbon is possible. If it can form a covalent bond then it qualifies the conditions to become a molecule. Therefore, CH4 is a molecule.
9. How many Sigma bonds are there in CH4?
CH4 has a total of four single bonds sharing between the central and terminal atoms. Every single item is also a Sigma bond which means CH4 has four Sigma bonds in its molecule.
10. Why does CH4 have a 109.5 bond angle?
The carbon atom in CH4 is bonded with four hydrogen atoms which means minimizing the repulsion of electrons in terminal atoms, the hydrogen atoms have to spread further away from each other which makes an angle of 109.5.
Conclusion
Methane Ch4 which is derived from natural resources or human-based activities is useful for us and become a source of many other important chemicals that are used in industrial products. Just like that methane CH4 is a colorless and odorless gas that is also found in natural gas.
The molecular geometry of methane CH4 is tetrahedral and the above article proved the formation of tetrahedral structure. What we found out is that CH4 is formed by one carbon and four hydrogen atoms single-bonded to each other. The molecule of methane uses 8 valence electrons to complete the octet shell of carbon and hydrogen.
The reason why methane is bound to have a tetrahedral structure is that the central atom does not have any lone pairs of electrons while the electrons present in the outer structure of this molecule repel each other and take positions as far away from each other as possible which makes a very stable tetrahedral structure of methane.
The reason why CH4 has a tetrahedral structure is that The molecule of methane is symmetrical in shape and the electronegativity differences between them show that it is a nonpolar bond.
