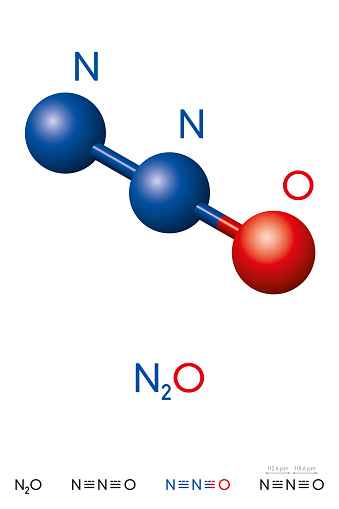
N2O molecular geometry is linear with a 180-degree bond angle. The N2O molecular geometry is covalently bonded by nitrogen (N) and oxygen (O) atoms. The valence electrons of N and O are five and six, respectively.
N2O Preparation Methods
There are several methods for making nitrous oxide. Here are a few ideas:
Heating ammonium nitrate in a large industrial reactor produces nitrous oxide and water vapor.
NH4NO3 ——–> 2H2O + N2O
Laboratory methods:
In the laboratory, nitrous oxide may be produced. N2O is produced by heating a solution of sodium nitrate and ammonium sulfate.
2 NaNO3 + (NH4)2SO4 ——-> Na2SO4 + 2N2O + 4 H2O
Urea, nitric acid, and sulfuric acid can also be used to create nitrous oxide.
2 (NH2)2CO + 2 HNO3 + H2SO4 → 2 N2O + 2 CO2 + (NH4)2SO4 + 2H2O
Ostwald process:
Nitrous oxide is produced by oxidizing ammonia using manganese dioxide and bismuth oxide as catalysts.
The Ostwald process is the name given to this procedure.
2NH3 + 2O2 ——> N2O + 3H2O
N2O may be made by a plethora of other reactions as well. Additionally, nitrous oxide is a large component of the atmosphere on Earth’s surface. The ppm value is 0.330.
Two natural processes known to create nitrous oxide are the processes of nitric and denitrification, which are both biological.
We now need to know about N2O’s Lewis structure, hybridization, and bonding in order to comprehend every other reaction involving N2O.
Summary
Heating sodium nitrate and ammonium sulfate solutions yields N2O. There is a significant amount of nitrous oxide in the Earth’s atmosphere. This approach is referred to as the Ostwald process.
N2O Lewis Structure
You should know how to draw a Lewis structure before getting into the Lewis structure of nitrous oxide.
Number of electrons of valance shell
To begin, we need to determine how many oxygen and nitrogen atoms’ valence shell electrons will be provided to the molecule.
-
Nitrogen has a valence shell of 5 electrons.
-
The N2O molecule contains two nitrogen atoms.
-
Nitrogen atoms provide five times two electrons each, which equals 10 electrons.
-
The valence shell of oxygen has six electrons.
-
There are a total of 16 electrons available thanks to all of the atoms.
-
Lone pairs in valence shells = 16/2 = 8 total electron repelling pairs.
Center atom of N2O
It is common for nitrogen to be the most likely candidate for the central position, as it has a greater ability to exhibit high valence than oxygen does.
As a result, one of the nitrogen atoms is placed in the center of the molecule, with the other nitrogen atoms surrounding it.
Charges on atoms
When drawing a molecule, it is important to keep the number of charges on atoms as low as possible, as this increases the molecule’s stability.
Shape of N2O molecule around a center atom
Two nitrogen atoms and one oxygen atom make up the N2O molecule. N2O’s most stable Lewis’s structure should be used to determine the molecular geometry.
Finding the molecular geometry is dependent on the number of single bonds and lone pairs that surround the central atom.
The N2O molecule’s linear form is due to the presence of two sigma bonds and zero lone pairs around the central atom (nitrogen).
The most stable N2O structure may be determined by drawing resonance structures of N2O. Nitrogen oxidation numbers in N2O are determined by looking at the structures that are the most stable. The N2O molecule’s structure is now complete.
In short
One oxygen and two nitrogen atoms make up the N2O molecule. Nitrogen molecules have one nitrogen atom in the center and the rest of their molecules circle that one. Molecule structure is determined by the number of bonds and lone pairs surrounding its core nucleus.
N2O resonance structures
Oxygen has a higher electronegativity than nitrogen. Oxygen’s negatively charged atoms are more stable than nitrogen’s negatively charged atoms. We may also remark that, compared to nitrogen, oxygen prefers electrons.
As a result of the oxygen atom’s positive charge, structure 3 has the greatest potential for N2O instability.
Despite its flaws, the second structure is stable. Structure 1 is the most stable resonance because oxygen holds the negative charge.
Summarize
In terms of electronegativity, oxygen has a greater value than nitrogen. Structure 3 has the highest potential for N2O instability because of the positive charge on the oxygen atom. The second structure, in spite of its shortcomings, is stable. Structure 1 is the most stable resonance because oxygen is holding the negative charge. "
Oxidation state of N2O
Nitrogen’s oxidation number in N2O is 1. One nitrogen atom is in a +2 oxidation state, whereas the other is in a zero oxidation state in reality. In terms of oxidation, oxygen has a value of 2.
Oxidation number from equation
Oxygen has an oxidation number of 2 in most cases. N2O contains two atoms of nitrogen. For example, nitrogen’s oxidation number is x. The N2O molecule has a net charge of zero.
Several oxidation numbers of each element in the molecule should have the same value.
x*2 + (-2) = 0
x = +1
To Summarize
Nitrogen is found in two atoms in N2O. It is well-known that oxygen has a reactivity of 2. Molecules of N2O have an electrical charge of 0. The oxidation numbers of every element in the molecule should be similar in value in several different ways. Nitrogen, for example, has an oxidation number of x.
Molecular Geometry of N2O
The molecular geometry of N2O is linear. Thus, this molecule belongs to the AX2 class. Linear electron and molecule geometry may be seen.
Electron geometry and molecular geometry are two terms that are often used interchangeably. While determining the structure, molecular geometry just considers the atoms, but electron geometry considers all of the electron pairs.
| Total Domains | Generic Formula | Picture | Bonded Atoms | Lone Pairs | Molecular Shape | Electron Geometry | Example | Hybridi-zation | Bond Angles |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AX | A-X | 1 | 0 | Linear | Linear | H2 | s | 180 |
| 2 | AX2 | X-A-X | 2 | 0 | Linear | Linear | CO2 | sp | 180 |
| AXE | A-X | 1 | 1 | Linear | Linear | CN° | |||
| 3 | AX3 | X-X-X | 3 | 0 | Trigonal planar | Trigonal planar | AlBr3 | ||
| AX2E | X-0-X | 2 | 1 | Bent | Trigonal planar | SnCl2 | sp2 | 120 | |
| AXE2 | X- A | 1 | 2 | Linear | Trigonal planar | O2 | |||
| 4 | AX4 | X-A-X-X-X | 4 | 0 | Tetrahedral | Tetrahedral | SiCl4 | ||
| AX3E | X-A-X-X | 3 | 1 | Trigonal pyramid | Tetrahedral | PH3 | sp³ | 109.5 | |
| AX2E2 | X-A-X | 2 | 2 | Bent | Tetrahedral | SeBr2 | |||
| AXE2 | X-A | 1 | 3 | Linear | Tetrahedral | Cl2 | |||
| To put it another way, lone pairs are included in electron geometry but are excluded from molecule geometry. Even though the N2O molecule has a linear structure, the atoms do not share electrons equally. The molecule’s charge density is not uniform. |
Because of this, it has a dipole moment and is categorized as polar.
Sum Up
N2O has a linear structure since it belongs to the AX2 class. Because of its dipole moment, the molecule’s charge density is not uniform. The atoms in the N2O molecule do not exchange electrons evenly, despite the fact that the molecule has a rather linear shape.
N2O Hybridization
In N2O, the nitrogen and oxygen atoms are both sp hybridized, while the oxygen atom is also sp3. The sp-hybridization of N2O may be explained by the fact that the terminal nitrogen is linked to another nitrogen by a triple bond. Hence, it is sp-hybridized.
It’s the same with the next nitrogen. A single connection connects the oxygen atom to the nitrogen atom, making it Sp3 hybridized. Another method of determining hybridization is by looking at the bonds.
Summing Up
N2O is composed of two sp hybridized nitrogen-oxygen atoms as well as one oxygen atom that is likewise sp3. Triple bonds between terminal nitrogen and another nitrogen may be the explanation for Sp-hybridization.
N2O Molecular Orbital Diagram
According to molecular orbital diagrams, a molecular orbital can be mixed together. The bond order of a chemical may be established using a MO diagram, giving us a notion of the length and stability of the bond.
Understanding the fundamentals of nitrous oxide’s molecular structure is all that is required to deduce its behaviour. Nitrogen’s atomic orbital (AO) is illustrated on the left, whereas oxygen’s AO is displayed on the right.
The molecular orbital of the compound NO may be seen in the centre. In the case of N2O, the left-hand side will have two nitrogen AOs. Atomic orbitals 2 and 3 will be arranged next to each other. Nitrous oxide will also have the same amount of oxygen in it as well.
Combine the AOs to generate the molecular orbital.
To Sum up
The AOs of nitrogen and oxygen may be shown side by side. All that is needed to determine NO’s behavior is an understanding of its fundamental structure.
Frequently Asked Questions
Following are some frequently asked questions related to N2O molecular geometry.
1. Is NO2 linear or bent?
However, if you remove 1 electron from the molecule, transforming it into NO2+, the molecule straightens out because of the lone electron loss. Nitrogen dioxide, on the other hand, is an AX2E species with a 134-degree angle. The lower angle is caused by the SF2 molecule’s extra lone pair.
2. Is NO2 a structure?
NO2 is a covalent molecule that has a nitrogen nucleus bound to an oxygen atom and another oxygen nucleus bound to another oxygen nucleus. Nitrogen dioxide is a reddish-brown gas with a density of 1.8 g/dm3 at ambient temperature.
3. Why does NO2 have sp2 hybridization?
When the bonding occurs, the two oxygen atoms will create a single and a double bond with the nitrogen atom. Three hybridized orbitals are needed to house two sigma bonds and one electron in nitrogen. As a result of this, sp2 hybridization occurs.
4. What is the molecular geometry of NO2 negative?
In terms of chemical properties, NO2 and CO2 are quite similar. Are NO2 and CO2 different in terms of their shape and size? Two N=O double bonds and no unpaired electrons make the 180° bond angle less resonant and linear, like in CO2, which has two N=O double bonds and one unpaired electron.
5. Is nh3 trigonal planar?
The nitrogen atom in ammonia is surrounded by four areas of high electron density (3 bonds and one lone pair). A tetrahedral arrangement is used. With an H-N-H angle of 106.7 degrees, the resultant molecular form is trigonal pyramidal in shape.
6. Is N2O ionic or molecular?
There are covalent bonds in N2O because the atoms share electrons to create their chemical bonds (nitrogen-nitrogen and nitrogen-oxygen). Because the bonds in N2O are created by sharing electrons, it is a covalent molecule.
7. Which is more polar between NO2 and N2O?
There is a discrepancy in the electronegativity of NO2 and N2O. Because of its bent structure, NO2 has a greater dipole moment than N2O, which has a linear structure.
8. What is the hybridization of N2O?
For example, the hybridization in N2O of the two nitrogen nucleotides is sp due to the fact that the terminal nitrogen has one bond and one electron pair, while the intermediate nitrogen has two bonding pairs. This is because oxygen has one sigma bond and three electrons that can be borrowed.
9. How do you find the hybridization of NO2+?
A lone pair of Sigma bonds surrounds the center atom, hence the hybridization will be SP. If the total number of Sigma bonds around the central atom is 2 and there is no lone pair, the resulting hybridization will be SP.
10. How do you identify N2O?
The chilled liquid form of nitrous oxide is clear and colorless. At its boiling point of -89°C, it has a density of 1.22 g/cm3. Aromatic and slightly poisonous gases are released as the mixture boils. Inhalation of the gas produces soporific effects (laughing gas).
Conclusion
It’s good to know that you now have some background information on the infamous “laughing gas!” The focus of this article is on the fundamental chemistry of nitrous oxide. The NO2 molecular geometry is linear. Many more responses and deeper understandings are easily accessible after reading this piece of content.
N2O has a molecular weight of 44.013 g/mol and is colorless. This substance has a boiling point of -88.48 °C and a melting point of -90.86 °C. A wide variety of applications call for the use of nitrous oxide, from rocket motor oxidizers to internal combustion engines. As a propellant, it is also utilized in air freshener canisters.
Related Articles
Nh3 lewis structure molecular
What Is The Molecular Geometry Of Pbr3