N2 molecular geometry is a straight line. N2 is monochrome, impartial, and violent gas. It is the greatest rich group in Earth’s air. A lone pair of electrons enclosed all nitrogen atoms. N2 Lewis building would contain two nitrogen atoms attached to fold by a triple bond.
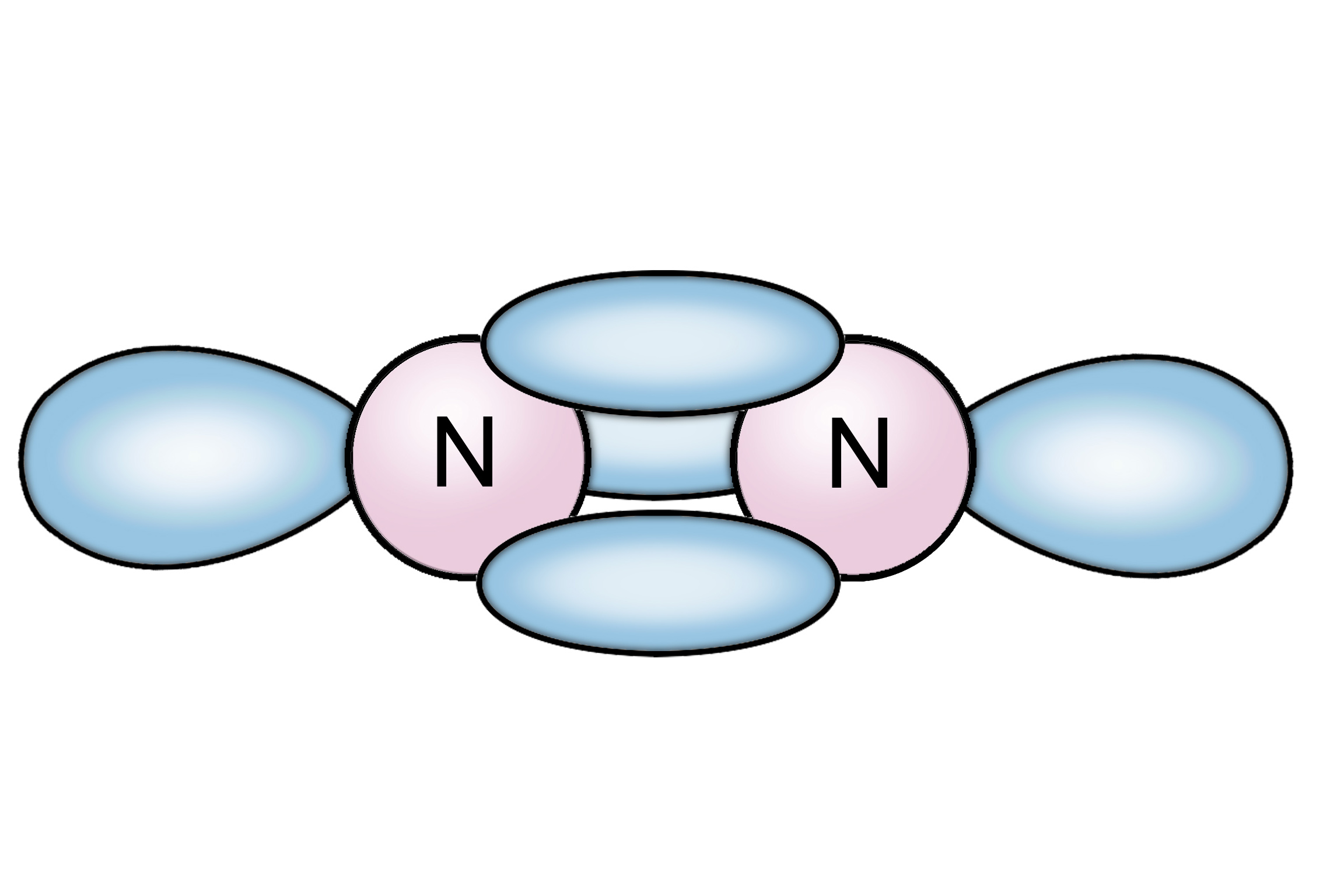
Molecular geometry for n2:
Nitrogen is a diatomic nonpolar particle with bond directions of 180 degrees. In the presence of a lined diatomic molecule, both atoms have an identical effect on the joint bonded electrons that type it into a nonpolar molecule.
| Name of Molecule | Nitrogen |
|---|---|
| Bond angle | 180 degrees |
| Molecular geometry of nitrogen | Linear |
| The polarity of N2 molecule | Non-polar |
| N2 valence electron | 10 |
N2 Lewis structure molecular geometry:
Stage by stage creation of Lewis structure:
In edict to be gifted to control the molecular geometry of a given complex, you need to first apply its Lewis structure.
Step 1: Total the valence electrons of atoms:
To draw Lewis structure we want to number out the number of valence electrons in single atoms as exposed in the table:
| Atom | Electronic configuration | Valence electrons |
|---|---|---|
| 7 N | 1s2 2s2 2p3 | 5 |
Amount of valence electrons in N2 = 5+5 = 10
Step 2: abode electron pairs among the atoms:
Together the atom has the same electronegativity rate, so there will be no vital atom in the assembly. We want to place 10 valence electrons in the building. Give the valence electrons by dots in a figure to all atoms alike 5 dots round all-atom.
Step 3: abode lasting electrons about the extra atoms:
Apply outlines to fix up the covalent bond amongst together the nitrogen atoms following each other. One line symbolizes one lone bond. All atoms finalize their octet (eight electrons per atom) by division three pairs of electrons that kind the delivery of 6 electrons in a bond. All nitrogen atoms cover two residual electrons which are named Ione pairs of electrons.
Lewis structure of N2 key points:
-
In the Lewis formation of N2, there is a tripartite bond amid two nitrogen atoms.
-
The molecule geometry of N2 is lined.
-
N2 is neutral, unscented, and cheap gas.
-
Quantity of electrons in the valence shell of nitrogen atom=5.
-
Every nitrogen atom is bounded by an Ion pair of electrons.
Lewis dot structure for a Nitrogen molecular geometry:
-
In the Periodic Table, Nitrogen is located in Group 5 through Age 2. So, each electronic structure of the group i.e. 2, 5, has five electrons in its furthest valence shell.
-
As each molecule N2, has binary atoms of Nitrogen. The whole number of electrons extant in the valence shell is 5 * 2 = 10e.
-
So, 10 valence electrons want to be decided in the building to display the chemical tie among two atoms of the Nitrogen molecule.
-
Currently, allot valence electrons about the atoms of N2.
-
Then you have 2 atoms of Nitrogen; allot the valence electrons by dots in a figure to every atom-like 5 dots around every atom. Use sign N to signify the atom.
-
Both the atoms have equal electronegativity; there will be no vital atom in the edifice.
-
Take repair of bonding and non-bonding electron couples that right affect the geometry of the Lewis group.
-
Now, fix up the covalent bond by script equally the Nitrogen atoms following each other and applying a line to signify the bond. Each bond displays two valence electrons. This bond is called a lone bond.
-
Display the lingering 3 electrons at the outside cross of both atoms.
-
To trail the octet law (eight electrons per atom), every Nitrogen atom wants 3 extra electrons i.e. 6 electrons to find the right structure.
-
Later making an ion bond among the atoms, together atoms have 6 electrons every. As each the octet law, quiet all atoms want two extra electrons to broaden its furthest shell.
-
At current, each atom has 7 electrons.
-
Lastly, after division three braces of electrons that type the supply of 6 electrons in a link, it is well-known as multiple covalent bonds.
Hybridization of nitrogen (N2):
- There are two kinds of bonds that are usually recycled in Chemistry, sigma (σ) and pi (π) bonds. Mutually the bonds support finding the type of hybridization by any starting head-to-head edge or when 2p detours edge.
-
Sigma bond is the main bond that is complete with extra atoms.
-
A pi bond is completely owed to the existence of a next or third bond.
-
The electronic shape of N2 atom (Z=7) is 1s2 2s2 2px 12py 12pz1.
-
There are three half-filled 2p detours in the valence shell of the nitrogen atom.
-
In creating the n2 molecule, a single of the three half-filled 2p detours of every nitrogen atom edges commonly beside the detain rich alliance to form a bond.
-
The residual dual half-filled 2p detours feel side-wise meeting with their similar concerns with 2p to form pi bonds.
-
So, two nitrogen atoms bond calmly done multiple bonds.
Uses of nitrogen:
-
Most alive kits want n2 to live. Nitrogen supports creatures raising, copying, and going diet into energy.
-
Nitrogen is vital to the chemical trade. It is castoff to type composts, nitric acid, nylon, and dyes.
-
Liquid nitrogen is used as a refrigerant for moving cuisine and icy drives.
What is nitrous oxide?
Nitrous oxide N2O is also well-known as giggling gas. It is vital for a change of medical requests because of its numbing use. It is difficult in water and works as a great oxidizer at complex fevers. It is neutral gas with faintly fragrant. It can source a downer result at higher focuses.
If a combination of nitrogen oxide with slight oxygen is gasped for a suitably long time. It crops agitated. Later nitrous oxide is too recognized as “chuckling gas”.
N2O Lewis structure:
In the N2o Lewis structure Nitrogen (N) and oxygen (O), molecules are covalently attached. The quantity of valence electrons in N and O is five and six singly. The total quantity of valence electrons in N2O is 16.
Is N2 polar or nonpolar?
N2 is a nonpolar molecule as of its lined geometrical formation and it is a diatomic molecule. As an end, together atoms have identical electronegativity and cut an equal amount of control and general molecule affect in a net-zero dipole moment creating it a nonpolar molecule.
General characteristics of nitrogen:
-
Nitrogen is current in the free municipal in the air as a main basic (78% by volume).
-
It is a nonmetal and deprived electrode of warmth and power.
-
Its mixes are covalent in Fauna.
-
N2 is a lazy gas in contrast with oxygen which is following the main structure of air.
-
Mineral mixes of nitrogen are not usually created as reserves.
-
In joint conditions, nitrogen is created in all living materials with, faunas and herbs in the form of proteins, urea, and amino acid.
Properties of n2:
-
The N2 Lewis structure displays two nitrogen atoms fused in a similar way to all others. It’s flawlessly symmetric.
-
Usually, small symmetric particles are nonpolar. The N2 Lewis structure shows that the N2 molecule is flawlessly symmetric. So, N2 is a nonpolar material.
-
Minor nonpolar materials are inclined to be gasses. We incline them to have low-temperature points. For the sample, N2 needs to be cool to about -200 ℃ or -320 ℉ to liquify it. The Earth does not grow this cold, and the air breaks filled with N2 gas.
Molecular Orbital Diagram of N2
Molecular detours are in particles where each particle has its electron shape in relation to a sigma bond and pi bond.
Giving to molecular detour model, it says about attractive flora, constancy direction, and the number of bonds in a particle.
When two detours are new, the result is even bonding molecular detours and when orbitals are deducted, it is called unstable anti-molecular bonding (*) which has extra vigor than the last one.
As the vigor level figure, the shape of N2 is σ1S2, σ 1S2, σ2S2, σ2S2, π2Px2, π2Py2, σ2Pz1
Advantages and Disadvantages of nitrogen inflated tires:
There are some advantages, and disadvantages of nitrogen inflated tires.
| Advantages | Disadvantages |
|---|---|
| Nitrogen is extra even than oxygen. … | Nitrogen rise is fairly expensive when likened to oxygen |
| Nitrogen recovers trip value. … | It wants to be measured extremely beside the zero rates of oxygen. |
| Nitrogen rises tire time. … | Keep of nitrogen full tires is also fairly delicate |
| A tires’ weight rises when it is bare to high fever | when you have complete nitrogen secret your tires, it is needed that you have to usage only nitrogen |
| Nitrogen decreases the successive fever at high haste and cargo of your tire. | The obtainability of nitrogen is also a subject occasionally as it is not simply offered universally. |
Summary:
Nitrogen seems like a neutral unscented gas. Non-flammable and non-fatal. Kinds the main serving of the air, but will not be funding life by himself. Used in diet treating, in removal air training and freezing schemes, and in forcing jet tires. Nitrogen is a section with atomic sign N, atomic figure 7, and atomic mass 14.01. In a nitrogen particle, a treble covalent bond is signified by three lines amid atoms of nitrogen. The bond direction is 180 degrees and there are 10 valence electrons. N2 is a nonpolar particle with lined geometry.
Frequently Asked Questions:
There are some FAQs related to N2 molecular geometry.
Q.1 Does N2 has bent geometry?
The geometry near nitrogen with three attached ligands is so trigonal pyramidal. Nitrogen will also hybridize sp2 when there are lone two atoms attached to the nitrogen (one single and one double bond). The resultant geometry is set with a bond angle of 120 degrees.
Q.2 Is N2 linear or non-linear?
The molecular geometry for this particle will be lined, with a bond angle of near 180∘.
Q.3 What Is an N2 molecule?
Molecular nitrogen (N2) is a much-shared chemical composite in which two nitrogen atoms are tightly bound together. Molecular nitrogen is a neutral, unscented, cheap, and slow gas at usual heats and weights.
Q.4 what is the bond angle of N2?
In a Nitrogen particle, multiple covalent bonds are signified by three lines amid two atoms of Nitrogen. The bond direction is 180 degrees and there are 10 valence electrons.
Q.5 Is N2 polar or nonpolar?
N2 is a nonpolar particle with lined geometry.
Q.6 Are O2 and N2 polar molecules?
Usually, bonds amid two of the similar atoms, such as nitrogen (N2) or oxygen (O2) have an equal supply of electrons, creating the atoms non-polar.
Q.7 Why is nitrogen trigonal planar?
In the single third donating structure of an amide, the lone pair on nitrogen gives electron mass to generate a pi bond amid carbon and nitrogen.
Q.8 what does N2 stand for in math?
Numeral 2 (N2)
Q.9 Is N2 an atom or molecule?
Nitrogen gas (N2) is a particle as the bond among the nitrogen atoms is a molecular bond. Water (H2O) is a molecular complex as it is material ready from more than one gentle of the part that is seized organized with molecular bonds
Q.10 Is N2 a bond length?
N2 designs. Best improved geometry, 6-311G, with bond directions and sizes exposed. The bond size is 1.09 Angstroms. The bond size is 1.09 of Angstroms and the angle is 180 degrees.
Q.11 Which out of N2 and H2O is polar and why?
Nitrogen (N2) is a homodiatomic particle and is consequently non-polar. On the other hand, the geometry of H2O is pointed or fixed with a bond direction of 104.5∘.
Q.12 what does N2 look like?
Nitrogen seems like a neutral unscented gas. Non-flammable and non-fatal.
Q.13 what type of hybridization is found in N2?
The atomic detour hybridization of nitrogen in N2 is sp-hybridized. Stating the crushed formal electron shape of nitrogen.
Q.14 what is the bond between N2 and N2?
2.5 And 3 singly.
Conclusion:
In the Lewis production of the N2 molecule, there is a creation of multiple covalent bonds signified by three lines amid two atoms of Nitrogen. The waste two 2p orbitals convert two π bonds and electrons creating a pair amid the nitrogen atoms will kind a sigma bond.
VSEPR model dons that molecular geometry reduces nausea amid the valence electrons. In the shape, it drives in a collective edict from lower to higher-order vigor level. To compute the method is Bond order= (Nb-Na)/2.
Related article:
N2 Lewis Dot Structure
N2O Molecular Geometry
N2 Valence Electrons