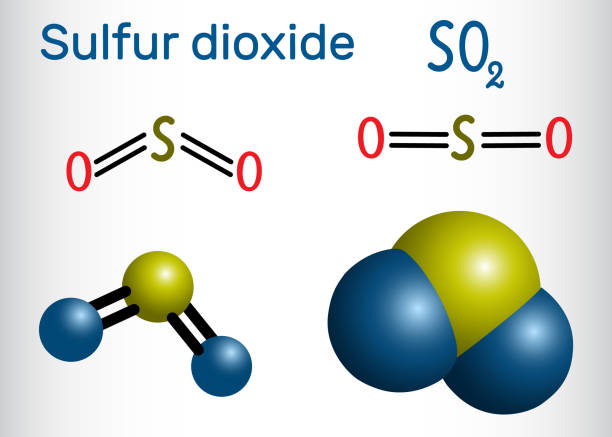
SO2 molecular shape is V-Shaped or Bent. SO2 molecular shape is same as the molecular geometry of Carbon Dioxide (CO2). When sulphur and oxygen are bonded together, they form sulphur dioxide, or sulphur dioxide, which is also called sulphur dioxide. SO2 is the formula’s shorthand.
SO2 Molecular Geometry
The molecular structure is formed by adopting a shape that minimizes the repulsions of electronic pairs. Carbon Dioxide’s molecular geometry is identical to SO2’s (CO2).
As a result, if we want to know the exact molecular shape of SO2, we need to know how sulphur and oxygen distribute electrons. Sulfur has six electrons and oxygen has four, with one electron being used for each bond at the outer level.
There are ten electrons in all, but they’re split up into five pairs. Four pairs are required to form bonds, so one pair is left alone. The two pairs of double bonds are combined into a single molecule.
We can conclude that the molecular shape of SO2 is V-shaped or bent because the single pair is not included in the shape’s description. This means that the original structure is not what we initially thought it was.
Summary
Because sulphur dioxide can decompose hydrocarbons into hydrogen halides, it is referred to as a reductant. Magnesium, carbon dioxide, and hydrogen sulfide can be oxidized by sulfur dioxide. Sulphur dioxide is an oxidizing agent because of this.
Difference of Electron Geometry Vs Molecular Geometry
Even though electron geometry and molecular geometry share many similarities, there are some important differences as well. The electron geometry can be associated with one or more molecular shapes, which is one of the most notable differences.
While the molecular geometry depends on other atoms that are either bonded to or have free pairs of electrons, it is the central atom’s structure of electrons that determines how the molecule looks.
SO2 Electron Geometry
Because of this, the electron geometry of SO2 is shaped like a trigonometric planner. At a 120-degree angle, three pairs of bonding electrons are placed in the plane. There was only one pair of doubles left, so the two doubles formed a bent shape.
SO2 Lewis structure
The eight valence electrons of sulphur must be arranged in order to form the Lewis structure of SO2. The formal charge of every atom must also be calculated in order to design the best Lewis structure.
As you are aware, sulphur and oxygen each have six valence electrons. There are two oxygen atoms in this case, so the total number of valence electrons is 18.
Sulfur will be in the middle, and oxygen will be on the outer edges. To form bonds, we’ll now put an electron pair between two atoms.
Let’s calculate the formal charges.
![]() 1. For Oxygen
1. For Oxygen
-
No. of valence electrons = 6
-
No of bonds = 2
-
Lone pairs = 2
-
So, Formal Charge = No. of valence electrons – No. of Bonds – 2 X (No. of lone pairs) = 6-2-(2×2) = 0
![]() 2. For Sulphur
2. For Sulphur
-
No. of valance electron = 6
-
No. of bonds = 2
-
Lone pairs = 2
-
So, Formal Charge = 6-2-(2×2) = 0
The octet will now be completed by the most electronegative element, O, completing the structure. Each oxygen atom will have a double bond and a lone pair attached to it.
The remaining valence electrons will be placed on the central atom to complete the structure. Using the formula (4+4) x2 = 16, we have four bond pairs and four lone pairs of electrons in this case. Because of this, there are only two valence electrons remaining. Sulfur’s atom will receive these electrons.
Summarize
It is also necessary to take into account the formal charge of each atom in order to design the most efficient Lewis structure. Each of the six valence electrons in sulphur and oxygen.
SO2 Bond Angle
An angle of 120 degrees is the bond angle of SO2. Sulfur atoms are covalently bonded to two oxygen atoms. It causes electron pairs to form a 120-degree angle by repelling each other.
Is SO2 Polar or Non-Polar?
As we can see from the Lewis structure, SO2 is asymmetrical due to the presence of a region with a different sharing ratio.
Because SO2’s molecular geometry is bent, the oxygen atoms at the top have less electronegativity than those at the bottom. As a result, SO2 can be classified as a polar molecule.
What is the Hybridization of Sulphur Dioxide?
![]() Sulfur dioxide undergoes hybridization of the sp2 type. A look at the sulphur nucleus is the first step in determining this. The central oxygen atom is joined to two other oxygen atoms to form SO2, which has the structural formula O=S=O.
Sulfur dioxide undergoes hybridization of the sp2 type. A look at the sulphur nucleus is the first step in determining this. The central oxygen atom is joined to two other oxygen atoms to form SO2, which has the structural formula O=S=O.
![]() The sigma and pi bonds between sulphur and the two oxygen atoms are both present. One lone pair may also be accommodated within the confines of the atom.
The sigma and pi bonds between sulphur and the two oxygen atoms are both present. One lone pair may also be accommodated within the confines of the atom.
![]() To get an idea of what it looks like in the ground state of sulphur, we can look at the outermost electron shell and the first and second shells.
To get an idea of what it looks like in the ground state of sulphur, we can look at the outermost electron shell and the first and second shells.
![]() The 3p portal has four electrons, and the 3s orbital has two paired electrons. This requires four unpaired electrons in order to form four bonds (with oxygen).
The 3p portal has four electrons, and the 3s orbital has two paired electrons. This requires four unpaired electrons in order to form four bonds (with oxygen).
![]() An excited state in sulphur occurs when one of the 3px electrons in the 3d orbital jumps to the 3d orbital. Electrons in the 3d orbital and the 3p orbital will be unpaired when this occurs.
An excited state in sulphur occurs when one of the 3px electrons in the 3d orbital jumps to the 3d orbital. Electrons in the 3d orbital and the 3p orbital will be unpaired when this occurs.
![]() Because of this difference in energy levels, it is not possible to combine electrons from the sigma bonds and lone pair. When hybridization occurs, a stable state is achieved.
Because of this difference in energy levels, it is not possible to combine electrons from the sigma bonds and lone pair. When hybridization occurs, a stable state is achieved.
![]() Through the process of hybridization, it is possible to combine two 3p orbitals with one 3s orbital. There are three sp2 hybrid orbitals in total.
Through the process of hybridization, it is possible to combine two 3p orbitals with one 3s orbital. There are three sp2 hybrid orbitals in total.
![]() Two hybrid orbitals have electrons that aren’t paired, while one hybrid orbital has a single pair. The oxygen atoms then form sigma bonds with the unpaired electrons.
Two hybrid orbitals have electrons that aren’t paired, while one hybrid orbital has a single pair. The oxygen atoms then form sigma bonds with the unpaired electrons.
![]() Pi bonds are formed by the interaction of 3d and 3p orbitals, which remain unchanged.
Pi bonds are formed by the interaction of 3d and 3p orbitals, which remain unchanged.
![]() The oxygen atom’s hybridization in this compound is also sp2, which is interesting.
The oxygen atom’s hybridization in this compound is also sp2, which is interesting.
In Short
The sp2 type of hybridization occurs in sulphur dioxide. This compound’s oxygen atom has sp2 hybridization, which is interesting. The electrons in Sp2 hybrid orbitals aren’t paired, whereas only one hybrid orbital has a pair.
SO2 Lewis Structure
-
A compound’s Lewis structure describes how electrons are distributed among its atoms.
-
The type of bond and the number of bonds that make up the compound can be determined using this structure.
-
Let’s now go over how to draw a Lewis structure step by step:
-
The first and most critical step is to determine the number of valence electrons in the molecule. While you’re at it, take care of the + and – signs.The “+” sign indicates that electrons are being lost, while the “-” sign indicates that electrons are being gained.
-
It’s now time to identify the core atom. The atom with the most bonding sites is the central one.
-
In the third step, you’ll create a skeleton structure with only single bonds.
-
After completing the octet of the atoms with the single bonds, our next step is to complete the octet with the remaining electrons. Electropositive atoms should always come after the electronegative ones.
-
In order to satisfy the octet rule for all atoms, it is necessary to use double or triple bonds.
-
Finally, make sure that all of the atoms have the lowest formal charges possible.
| Total domains | Generic formula | Bond pairs | Lone pair | Molecular shape | Electron geometry |
|---|---|---|---|---|---|
| 1 | AX | 1 | 0 | Linear | Linear |
| 2 | AX2 | 2 | 0 | Linear | Linear |
| 2 | AXE | 1 | 1 | Linear | Linear |
| 3 | AX3 | 3 | 0 | Trigonal planar | Trigonal planar |
| 3 | AX2E | 2 | 1 | Bent | Trigonal planar |
| 3 | AXE2 | 1 | 2 | Linear | Trigonal planar |
| 4 | AX4 | 4 | 0 | Tetrahedral | Tetrahedral |
| 4 | AX3E | 3 | 1 | Trigonal planar | Tetrahedral |
| 4 | AX2E2 | 2 | 2 | Bent | Tetrahedral |
| 4 | AXE3 | 1 | 3 | linear | Tetrahedral |
SO2 Molecular Orbital Diagram
A molecular orbital diagram shows us how the atomic orbitals of two different atoms can combine to create a new orbital structure.
We can now use this information to determine the bond order, length of the bond, and strength of any given compound’s bond. On the left-hand side of this MO, we can see the AO of sulphur, which comes from the right-hand side, combining.
This shows that the orbitals have been filled in accordance with the correct rule. Additionally, there are some non-bonding orbitals in the vicinity. Additionally, the SO2 antibonding orbitals are void. SO2’s molecular orbital diagram can be summarised with this statement.
Key Points to Consider When drawing the SO2 Molecular Geometry
![]() The SO2 molecular structure can be drawn in three steps. Sketching the molecular geometry of the SO2 molecule is the first step; the second step is to calculate the lone pairs of electrons in central sulphur and terminal oxygen atoms; and the third step is to give perfect notation for SO2 molecular geometry in the correct manner.
The SO2 molecular structure can be drawn in three steps. Sketching the molecular geometry of the SO2 molecule is the first step; the second step is to calculate the lone pairs of electrons in central sulphur and terminal oxygen atoms; and the third step is to give perfect notation for SO2 molecular geometry in the correct manner.
![]() In a specific geometric way, the SO2 molecular geometry shows the number of valence electrons and bond electron pairs. Valence Shell Electron Pair Repulsion Theory (VSEPR) and molecular hybridization theory can then be used to predict the SO2 molecule’s geometry, which is determined by how far apart electrons are in a specific molecular structure.
In a specific geometric way, the SO2 molecular geometry shows the number of valence electrons and bond electron pairs. Valence Shell Electron Pair Repulsion Theory (VSEPR) and molecular hybridization theory can then be used to predict the SO2 molecule’s geometry, which is determined by how far apart electrons are in a specific molecular structure.
![]() Once you’ve added their bond polarities, you can compute the S-O double bonds’ strength (dipole moment properties of the SO2 molecular geometry).
Once you’ve added their bond polarities, you can compute the S-O double bonds’ strength (dipole moment properties of the SO2 molecular geometry).
![]() Because the SO2 molecule has a bond dipole moment that pulls the electron cloud to the two sides of its bent geometry, the sum of the (S-O) double bonds in the sulphur dioxide molecule is zero because all two (S-O) double bonds have the same size and polarity, and because all two (S-O) double bonds have equal polarity, their sum is zero, and the SO2 molecule is classified as nonpolar.
Because the SO2 molecule has a bond dipole moment that pulls the electron cloud to the two sides of its bent geometry, the sum of the (S-O) double bonds in the sulphur dioxide molecule is zero because all two (S-O) double bonds have the same size and polarity, and because all two (S-O) double bonds have equal polarity, their sum is zero, and the SO2 molecule is classified as nonpolar.
![]() SO2 molecule has a 119-degree bond angle between O-S-O and O-S-O. Sulfur and oxygen atoms have different electronegativity values; oxygen’s pull on the electron cloud is stronger than sulfur’s. However, in a V-shaped or bent geometry, the S-O bond polarity is not cancelled.
SO2 molecule has a 119-degree bond angle between O-S-O and O-S-O. Sulfur and oxygen atoms have different electronegativity values; oxygen’s pull on the electron cloud is stronger than sulfur’s. However, in a V-shaped or bent geometry, the S-O bond polarity is not cancelled.
![]() Consequently, its molecular structure has a dipole moment that can never go back to zero. Because of the unequal distribution of negative and positive charges in the V-shaped or bent geometry, the SO2 molecule has a nonzero dipole moment.
Consequently, its molecular structure has a dipole moment that can never go back to zero. Because of the unequal distribution of negative and positive charges in the V-shaped or bent geometry, the SO2 molecule has a nonzero dipole moment.
To Summarize
There are three steps to drawing the molecular structure of SO2. The geometry of SO2 can be predicted using VSEPR and molecular hybridization theory, both of which are based on the Valence Shell Electron Pair Repulsion Theory. There are an unequal number of negative and positive charges in the SO2 molecule, which makes it nonpolar.
SO2 electron and molecular geometry
The VSEPR theory states that the SO2 molecule has linear molecular geometry. With two S-O double bonds between the sulphur and the two oxygen atoms that surround it, In the bent SO2 molecular geometry, the O-S-O bond has a 119-degree angle.
In order to form the SO2 molecule, it contains two oxygen atoms in the V-shaped or bent form, as well as two corners with one lone pair of electrons on the central sulphur.
In the SO2 molecular geometry, there are two S-O double bonds. The V-shape or bent structure is maintained after linking the two oxygen atoms and one pair of electrons on the sulphur atom in the V-shape or bent form.
The two terminal S-O double bonds and one lone pair of electrons on the sulphur atom have remained in the SO2 molecular geometry due to the V-shaped or bent nature of the molecule.
It has a V-shape or bent electron geometry because SO2 has only one pair of electrons. So2, on the other hand, has one single pair of electrons on the sulphur of its molecular geometry and appears V-shaped or bent. The asymmetrical geometry of the SO2 molecule is to blame. Because of this, the SO2 molecule is polar.
Sum up
We’ll use an electron pair to join two atoms together to form a bond. Sulfur will occupy the centre, while oxygen will occupy the periphery.
Frequently Asked Questions
Following are some frequently asked questions related to SO2 molecular shape.
1. Is SO2 bent or trigonal planar?
Trigonal planar electron-domain geometry characterizes SO2. That’s because sulphur has three electron domains: it forms two single bonds with two oxygen atoms, and it has one non-bonding lone pair of electrons.
2. Is SO2 is a bent molecule?
When it comes to carbon dioxide, there are no “lone pairs” on it, but when it comes to sulphur, there is one. This is why the shape of SO2 is so bent.
3. What is the bond angle in SO2?
Because of a double bond between S and O, the angle is nearly 120 degrees. Sulfur dioxide has a sp2 hybridized structure. As a result of this arrangement, one of the central sulphur atoms is bonded twice to one of its neighbours, while the other is linked to the other. It has a 119-degree bond angle.
4. Is no3 trigonal planar?
A single central atom is flanked by three identically bonded oxygen atoms that are all situated in the same one-dimensional plane as the central atom. Nitrate is a three-electron compound with no lone pairs of electrons. As a result, the molecular geometry of NO3—is trigonally planar with a slight bend.
5. Is SO2 ionic or covalent?
SO2 is a covalent molecule. Covalent bonds are formed when the electronegativity of the two atoms differs just enough to cause the electron bonds to be shared.
6. Is ClF3 trigonal planar?
The Cl atom in ClF3 has five electrons surrounding it. Two nonbonding and three bonding components. If you draw the molecule’s Lewis structure, you should see this. The molecule will take on a trigonal bipyramid shape if it has five electron groups surrounding the central atom.
7. What type of compound is SO2?
The inorganic compound sulphur dioxide (SO2) is a heavy, colourless, and poisonous gas. Sulfuric acid manufacturing produces a massive amount of this intermediate product. In the same way that the smell of just-lit matches is recognisable, the pungent, irritating odour of sulphur dioxide is also.
8. Is PH3 an sp2?
According to the PH3 molecule’s Lewis structure and Steric number rule, the hybridization is sp3. However, the PH3 molecule does not have any hybridization. It’s a Drago molecule, thus the name. No hybridization occurs in Drago molecules, which also have the smallest bond angles.
9. Is SO2 empirical or molecular?
However, we already know that sulphur dioxide gas has the molecular formula SO2. The molecular formula for SO2 is SO2. Dividing by 2 shows that this formula can be simplified. B2H6 is a molecular formula because it can be divided and transformed into an empirical formula.
10. Is SO2 an oxidizing agent?
Because sulphur dioxide can decompose hydrocarbons into hydrogen halides, it is referred to as a reductant. Magnesium, carbon dioxide, and hydrogen sulphide can be oxidised by sulphur dioxide. Sulphur dioxide is an oxidising agent because of this.
Conclusion
In this article, we’ve discussed SO2’s molecular geometry, electron geometry, Lewis structure, bond angle, and polarity, among other things (Sulfur Dioxide). If there is any information that has been omitted or if you have further questions, please do not hesitate to ask.
Related Articles
Predict The Molecular Shape Of Methane
Sbr2 Molecular Geometry
V-Shaped Recovery