H lewis structure has a linear structure. This is because the shared pair of electrons is shown as two dots between the two H symbols (H : H).
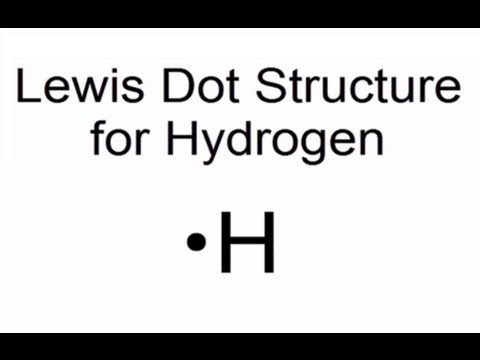
 H Lewis Structure
H Lewis Structure
Because it only has one valence electron, the hydrogen atom is represented by the symbol H•. Lewis electron-dot structures are a type of diagram that may be used to depict the structures of molecules that are bound together by covalent bonds.
Between the two H symbols are two dots, which represent the electrons that are shared by the two H symbols (H:H). When two atoms are connected by the sharing of a single pair of electrons, this is referred to as a single covalent bond.
-
It is also possible to express a single covalent connection by placing a dash between the two symbols (H–H). Structure formulae are formulae that illustrate the organization of atoms in a molecule and express covalent bonds between atoms using dashes to describe the arrangement of atoms.
-
During the formation of ions, they adhere to the octet rule by either losing or acquiring electrons to attain the electron configuration of the closest noble gas, which is determined by the atomic number.
-
When nonmetal atoms join together to create a covalent bond, they share electrons in such a manner that each of the atoms participating in the link may achieve a noble-gas electron configuration, this is known as electron sharing.
The electrons that are shared are “counted” for each of the atoms that are participating in the sharing. Each atom of hydrogen (H2) can achieve the electron configuration of helium, which is an extremely rare noble gas that has just two electrons, thanks to a shared pair of electrons. While this is not always the case for atoms other than hydrogen, sharing electrons will often supply each atom with eight valence electrons.
SUMMARY
Structures of Lewis electron-dots in covalent compounds demonstrate the bonding between the molecules. It is possible to denote covalent bonds between atoms using either dots ( : ) or a dash (-).
 Writing Lewis Structures With The Octet Rule
Writing Lewis Structures With The Octet Rule
To depict electronic structures of valence, draw Lewis symbols (for atoms and monatomic ions) and Lewis structures (for molecules) (for molecules and polyatomic ions). A Lewis structure is denoted by the presence of lone pairs or unpaired electrons, as well as the presence of single, double, or triple bonds, which indicate the location of valence electrons around each atom.
In the case of molecules with odd electrons (free radicals), electron-deficient molecules, and hypervalent molecules, the octet rule is violated in a few cases.
-
You should remove one electron per positive charge when dealing with anions.
-
If there’s a rule of thumb, the most electronegative component should go in the center of the design. There should only be one connection between each atom and the atom in the centre of your design (one electron pair).
-
The center atom should get all of the remaining electrons.
-
In order to get octets wherever possible, rearrange the electrons of the peripheral atoms so they make numerous bonds with the core atom.
Summary
As a result of the hydrogen atom having just one electron, hydrogen may readily escape from the electron and transform into an ion of positive charge. Following the donation of electrons by the hydrogen atom, the final shell of hydrogen is devoid of electrons. Consequently, the valence electron of a hydrogen ion is a negative number.
 Covalent Bonds And Lewis Diagrams
Covalent Bonds And Lewis Diagrams
The most straightforward example to consider is hydrogen (H), which is the smallest element in the periodic table with only one proton and one electron, making it the most basic element to understand.
For hydrogen to become stable, it must attain a complete valence level, similar to that of the noble gas nearest to it in the periodic table, helium (He). These are exceptions to the octet rule because they only require two electrons to achieve a complete valence state, whereas the rule requires eight electrons.
Two hydrogen atoms can join together and share each of their electrons to form a ‘covalent bond.’ It is possible to consider the shared pair of electrons as belonging to either atom and therefore each atom, such as He, now has two electrons in its valence level, rather than one. The molecule formed as a result of this reaction is H2, which is the most prevalent molecule in the entire universe.
 Lewis structure Of hydrogen
Lewis structure Of hydrogen
This is the procedure that results in the formation of the H2 molecule. Two H atoms, each of which contributes an electron, pool their electrons to form a pair. A single covalent bond’ is what this is referred to as. Take note of how the two electrons can be located in an area of space between the two atomic nuclei, as shown in the diagram.
H:H or H—H is the Lewis formalism that is used to represent the H2 molecule. While the former, referred to as a ‘Lewis dot diagram,’ shows a pair of shared electrons between the atomic symbols, the latter, referred to as a ‘Lewis structure,’ shows a pair of shared electrons that form a covalent connection with the help of a dash. It is also possible to show more sophisticated compounds in this manner.
 How Does Octet Rule Work?
How Does Octet Rule Work?
When an atom prefers to have eight electrons in the outermost shell, it is known as the “octet rule.” More stable compounds are formed when atoms have less than eight electrons. We do not address d or f electrons while discussing the octet rule. When applied to elements in the main group (those not in the transition metal or inner-transition metal block), the octet rule only affects s and p electrons, making it a useful tool.
-
An element’s maximum positive and negative valences are often eight. This is known as Abegg’s rule, which was developed by Richard Abegg in 1904. These rules were re-used by Gilbert N.
-
Lewis in 1916 when he developed the “octet rule” for his cubical theory. Atoms will respond to achieve the highest feasible level of stability. If an octet is complete, all of its orbitals will be filled.
-
As a result, a process that improves the stability of the atoms will produce heat or light energy as the atoms become more stable. When eight electrons encircle the atom, a stable configuration is achieved.
-
Each of the eight electrons in this octet can be made up of one or more electrons that are shared. This process of bond formation continues until an octet of electrons has been formed in an atom.
The valence level of noble gases like He, Ne, Ar, Kr, etc., is filled with as many electrons as possible, making them stable. All noble gases except helium have eight electrons at their entire valence level, except for helium, which contains two electrons.
 Exceptions To The Octet Rule
Exceptions To The Octet Rule
Lewis structures of many covalent compounds feature core atoms with fewer than eight electrons. Three types of molecules make up these compounds:
-
Odd-electron molecules have an unpaired electron because they have an odd number of valence electrons.
-
Electron-deficient molecules feature a core atom that lacks the required number of electrons to form a noble gas configuration.
-
The core atom of a hypervalent molecule has more electrons than it needs to function as a noble gas.
Summary
A valence level packed with electrons, like in noble gases, is achieved by other elements in the periodic table reacting and forming bonds. Atoms tend to build molecules and compounds by combining in groups of eight, which we call the “octet rule.”
 Covalent Bonding Through The Use Of Lewis Dot Symbols
Covalent Bonding Through The Use Of Lewis Dot Symbols
Covalent bonding is based on the sharing of electrons that allow atoms to “stick” together. If both atoms can acquire a full s2np6 configuration, the attractive forces will greatly dominate the repulsive forces at some intermediate distance, usually a little longer than 0.1 nm or, if you prefer, 100 pm.
It is precisely this type of behavior that Lewis documented in his octet rule. A covalent compound’s structure, stoichiometry, and characteristics are all influenced by its valence electron configurations.
When it comes to electrons, chlorine is one shy of an octet, because it has seven valence electrons instead of the eight required. To complete their valence shells, two chloride atoms can produce Cl2 by sharing their unpaired electrons in a covalent connection.
The octets of chlorine atoms have been added. Electrons on each chloroplast are known as “lone pairs,” except the electrons that form a “bonded pair” between them. Covalent bonds are not formed by lone pairs.
Coordinate covalent bonds are formed when both electrons in a covalent bond originate from the same atom. This sort of bonding is discussed in Section 5.4 about atoms that have less than eight electrons.
Summary
Hydrogen ion (H2O) is represented by the Lewis structure on the right. The oxygen atom’s octet is now complete, consisting of four bonded pairs and two lone pairs. It’s also worth noting that hydrogen now possesses two electrons in its valence shell thanks to the hydrogen-oxygen connection. Chemists use a single line to represent a bonding pair.
 Importance Of Hydrogen
Importance Of Hydrogen
There is more hydrogen in the Universe than in any other element. Hydrogen accounts for approximately 90% of all atoms. It is an essential component of the human body’s fluids that transports and eliminates toxins and waste.
Our joints were lubricated and ready to carry out their duties thanks to hydrogen. As a result, even the immune system is protected. Because of this, it is correct to claim that hydrogen is a crucial component of life on Earth and can be found virtually everywhere.
In Chemistry, hydrogen is the heaviest and lightest element. Are you aware that it happens most frequently in a gaseous state? It is a tasteless, colorless, and odorless substance that is extremely combustible.
Summary
In terms of molecular weight, it is 100797 g/mol. In 1671, Robert Boyle, using diluted hydrochloric acid to dissolve iron, discovered that it was a distinct gas. Protium, deuterium, and tritium are all hydrogen isotopes with the same atomic number but distinct atomic weights.
 About Hydrogen
About Hydrogen
 Fast Facts
Fast Facts
| Atomic number | (Z) 1 |
| Group | group 1: hydrogen and alkali metals |
| Period | period 1 |
| Block | s-block |
| Electron configuration | 1s1 |
| Electrons per shell | 1 |
| Phase at STP | gas |
| Melting point | (H2) 13.99 K |
| Boiling point | (H2) 20.271 K |
| Density | 0.08988 g/L |
| Triple point | 13.8033 K, 7.041 kPa |
| Critical point | 32.938 K, 1.2858 MPa |
| The heat of fusion | (H2) 0.117 kJ/mol |
| The heat of vaporization | (H2) 0.904 kJ/mol |
| Molar heat capacity | (H2) 28.836 J/(mol·K) |
Hydrogen is a diatomic gas with the formula H2. Not only is it non-toxic but it is also extremely flammable. Hydrogen is the most abundant chemical in the universe, making up nearly 75% of all matter. Most hydrogen on Earth is in the form of water or organic molecules. Each atom of 1H has one proton, one electron, and no neutrons.
Protons, the hydrogen nuclei, formed in the first second following the Big theory. It was 370,000 years later when the plasma had cooled sufficiently for electrons to remain bonded to protons, that neutral hydrogen atoms appeared throughout the cosmos.
The H+ cation is a proton (symbol p), but in aqueous solutions and ionic compounds, it is charged by surrounding polar molecules or anions. Because the Schrödinger equation can only be solved analytically for hydrogen, its energetics and chemical bonds have been studied extensively in the development of quantum mechanics. Acids on metals created hydrogen gas for the first time in the early 1600s.
Summary
Acid-base interactions frequently entail proton exchange between soluble molecules, hydrogen is vital. When used in ionic compounds, hydrogen can be either a negatively charged (anion) or positively charged (cation) species, represented by the symbol H+.
 Hydrogen Properties
Hydrogen Properties
 Hydrogen (ortho and para)
Hydrogen (ortho and para)
Ortho and para molecular hydrogens are known. The protons’ magnetic interactions differ depending on their spinning movements.
-
Ortho-hydrogen has both protons’ spins aligned in the same direction, parallel. Para-spins hydrogen is antiparallel, oriented in opposing directions.
-
The magnetic characteristics of atoms are determined by spin alignments. Normally, there are no conversions between ortho and para molecules, hence ortho-hydrogen and para-hydrogen are two separate variations of hydrogen. Under specific situations, the two forms may interconvert.
-
There are various approaches to achieve this equilibrium. One technique is to add catalysts (such as activated charcoal or paramagnetic compounds), another is to apply an electrical discharge or heat the gas to a high temperature.
The periodic table begins with hydrogen. The main role of hydrogen in the human body is hydration. Water contains hydrogen and oxygen and is absorbed by the body’s cells. So it is a vital element utilized in our bodies, fuel, military weaponry, etc. This article discusses the role of hydrogen in the human body.
 Hydrogen Reactivity
Hydrogen Reactivity
Hydrogen molecules dissociate into two atoms (H2 2H) when a force equal to or higher than their dissociation energy (i.e., necessary to break the bond that binds the atoms together) is applied.
This energy is produced by contacting the gas with a white-hot tungsten filament or by creating an electric discharge in the gas. Atomic hydrogen created at low pressure has a large lifetime of —0.3 seconds at 0.5-millimeter mercury pressure.
Atomic hydrogen is flammable. It reacts with most elements to make hydrides (e.g., sodium hydride, NaH) and reduces metallic oxides to form the metal. The energy released by this reaction heats the surfaces of metals that do not mix with hydrogen to create stable hydrides (e.g., platinum).
 H-bond
H-bond
In certain covalently linked hydrides, a hydrogen atom is hydrogen-bonded to two distinct electronegative atoms. The strongest hydrogen bonds are formed by fluorine (F), oxygen (O), and nitrogen (N).
Hydrogen connects two fluorine atoms in bifluoride ion (HF2). Each oxygen atom in ice is surrounded by four other oxygen atoms, with hydrogen atoms between them. When ice melts, some hydrogen bonds break, and the structure collapses. In biology, hydrogen bonding is vital in defining a molecular configuration.
Proteins, for example, have helical (spiral) structures bound together by hydrogen bonds. HF, H2O, and NH3 have substantially higher boiling temperatures than their heavier equivalents, HCl, H2S, and phosphine (PH3). The greater the boiling temperature, the more energy is available to break apart hydrogen bonds and allow vaporization.
 Hydrogen Isotopes
Hydrogen Isotopes
Francis William Aston discovered in 1927 that the line for hydrogen matched to an atomic weight of 1.00756 on the chemical scale. Averaging the combining weights of hydrogen compounds, this number was more than the expected experimental error, 1.00777.
Urey. In 1931, Urey and two colleagues discovered deuterium in the residue of a liquid hydrogen distillation. Because deuterium is a rarer element than water, it is concentrated in the residue when an electrolyte solution such as sodium hydroxide is electrolyzed. When a solution is reduced to 0.00001 of its original volume, it yields almost pure deuterium oxide (D2O).
Summary
Others demonstrated that the disparity might be eliminated by assuming the presence of a mass 2 hydrogen isotope with one 2H (or D) atom for every 4,500 1H atoms. For example, the difference in vapor pressures of hydrogen (H2) and deuterium (HD) may be used to separate these compounds by distillation of liquid hydrogen, according to US chemist Harold C.
 Applications
Applications
Ammonia produced as a result of this process is a primary source of protein for humans. hydrogenation is a method for transforming unsaturated oils into saturated ones. The most common use is to make margarine.
-
Petrochemical industry: Fossil fuels are “upgraded” by using a large amount of hydrogen (H2). Hydrodealkylation, hydrodesulfurization, and hydrocracking are three of the most important users of H2 in the industry. Several chemical processes fall under the hydrogenolysis category, which refers to the breakage of carbon-hydrogen bonds. In this regard, the process of removing sulfur from liquid fossil fuels is instructive:
-
Hydrogenation: It is common to practice hydrogenating a wide variety of materials on a big scale. The Haber-Bosch Process uses just a small percentage of the industry’s total energy budget to hydrogenate N2 and create ammonia. Hydrogenation of carbon dioxide results in the production of methanol. Hydrochloric acid production also relies on this supply of hydrogen. In the process of turning certain ores into metals, H2 is employed as a reducing agent.
-
Coolant: In power plants, hydrogen is utilized as a coolant in generators because of its light diatomic molecules, which have a variety of advantageous features. These properties include low density, low viscosity, and the greatest specific heat and thermal conductivity of all gases.
-
Semiconductor industry: Stabilizing the characteristics of amorphous silicon and amorphous carbon by saturating the broken (“dangling”) bonds, hydrogen is used. Electron donors in different oxide materials may use it as a source of electrons.
-
Rocket propellant: Cryogenic fuel in liquid-propellant rockets, such as the main engines of the Space Shuttle, is composed of liquid hydrogen and liquid oxygen.
Summary
Both liquid hydrogen and compressed hydrogen gas at any practical pressure have much lower energy density per unit volume than traditional fuel sources, even though the energy density per unit fuel mass is greater. It has been frequently considered in the context of energy, as a viable medium for transporting energy across the economy in the future.
 Hydrogen As Energy Carrier
Hydrogen As Energy Carrier
Because there is no naturally occurring supply of hydrogen in usable proportions, it cannot be used as a source of energy or fuel for burning. Nuclear fusion of hydrogen is the source of the Sun’s energy, but this process is difficult to manage on Earth.
Because the energy required to create atomic hydrogen, whether from solar, biological, or electrical sources, is more than the energy gained from burning it, atomic hydrogen serves as a kind of energy storage. It is possible to get hydrogen from fossil fuels (such as methane), but this is not a long-term solution.
When producing hydrogen from fossil fuels, for example, CO2 sequestration might be followed by carbon capture and storage. Using hydrogen as a fuel for transportation would produce some NOx emissions but no carbon emissions and be a reasonably clean fuel.
Infrastructure expenses for a full hydrogen economy conversion would be enormous. Internal combustion engines can’t compete with the efficiency of fuel cells when it comes to turning hydrogen and oxygen into energy.
Frequently Asked Questions - FAQs
People asked many questions about hydrogen. We discussed a few of them below:
 Hydrogen is either H2 or H, but which is it?
Hydrogen is either H2 or H, but which is it?
In the cosmos, hydrogen is the most common element with an atomic number of one. The molecular formula for hydrogen is H2, and it has a molar mass of one. The atomic number 1 of hydrogen, abbreviated H, is that of the lightest element. Molecular formula H2 is a gas having an odorless, colorless, tasteless, and extremely combustible vapor.
 What is the purpose of hydrogen as a fuel?
What is the purpose of hydrogen as a fuel?
Because of hydrogen’s ability to power fuel cells in zero-emission cars, its domestic production potential, and the fuel cell’s quick filling time and high efficiency, it’s gaining attention as an alternative transportation fuel. Internal combustion engines may also use hydrogen as a fuel source.
 What is hydrogen’s role in the natural world?
What is hydrogen’s role in the natural world?
A low-emission, environmentally friendly, greener, and more sustainable energy system relies heavily on hydrogen. Hydrogen’s combustion product is water and a little number of nitrogen oxides.
 How many valence electrons does H have?
How many valence electrons does H have?
The Periodic Table places hydrogen in the first column. The electron configuration of hydrogen is 1s1 since it is the initial element. The valence shell of this atom has just one electron.
 Why is H not a cation, but rather an anion?
Why is H not a cation, but rather an anion?
In the periodic table, hydrogen is sometimes positioned above the halogens since it is nonmetal and produces H- (hydride anions). H2 dihydrogen is also formed by hydrogen, just as halogens such as chlorine and bromine. While the halogens are similar, hydrogen is distinct. In comparison to halogens, hydrogen has a far lower affinity for electrons.
 In what way does hydrogen play an important role in the star’s existence?
In what way does hydrogen play an important role in the star’s existence?
When stars form surrounding nebulae, hydrogen plays an important part in their formation. Stars are created from vast clouds of hydrogen. A chain reaction from the collision of atoms eventually causes the hydrogen to heat up and combust.
 Is there an H ion?
Is there an H ion?
It’s the nucleus of hydrogen that’s been split from its electron, and it’s known as an “ion.” The nucleus of hydrogen is made up of a proton, a particle with a single unit of positive electric charge. For this reason, the sign H+ has come to symbolize a proton in chemistry and other fields.
 Who was the first person to discover the existence of hydrogen?
Who was the first person to discover the existence of hydrogen?
Henry Cavendish, an English physicist, made the historic discovery of hydrogen in 1766. For many years before its official classification as an element, scientists had been working on ways to create hydrogen. In 1671, while working with iron and acids, Robert Boyle developed hydrogen gas, according to written sources.
 What type of bond is H2?
What type of bond is H2?
Covalent molecules made up of only one kind of atom, such as hydrogen gas (H2), is nonpolar because the hydrogen atoms share their electrons in the same proportion.
 What is the process through which hydrogen is converted into energy?
What is the process through which hydrogen is converted into energy?
Hydrogen fuel cells generate energy by mixing atoms of hydrogen and oxygen to form hydrogen gas. An electrochemical cell, similar to a battery, is used to generate electricity, water, and tiny quantities of heat by reacting hydrogen with oxygen over the cell’s surface.
Conclsuion
The electrons in the covalent link between the hydrogen atoms are represented by the two dots that connect the hydrogen atoms. It is a graphical representation of the outermost shell of electrons, also known as valence electrons, and any potential covalent bonds within an atom or molecule, and it is used to illustrate the Lewis Dot Structure.
In addition to being negatively charged, these valence electrons are also attracted to the positively charged nucleus, which is composed of neutrons and protons. Keep in mind that electrons are continually flowing around the nucleus and are not rooted in a single location as depicted in a 2D structural representation. Also, core electrons are equal to total electrons minus valence electrons, which is a valid statement. This likewise yields the same result as the previous method. As an example, hydrogen contains a total of one electron in its nucleus.
Related Articles
https://howtodiscuss.com/t/hydrogen-peroxide-burn/115350