Hydrogen Lewis dot structure. The two dots together between the Hydrogens define the electrons in the covalent bond between the hydrogen atoms.
How many Lewis dots are accumulated in hydrogen?

Two dots.
The two dots between the hydrogen atoms represent the covalent electrons between the hydrogen atoms.
Lewis Dot Diagram:
The Lewis point structure is like a simplified electron energy level model. The Lewis structure contains an element symbol with a dot representing an electron. The only electrons shown are external energy levels or valence electron electrons.
The electrons are individually placed around the element symbol in the clockwise or counterclockwise direction and are grouped in pairs as more electrons are added.
Hydrogen covalent bond:
The Lewis point structure can also represent an intramolecular bond atom. The two points between the hydrogen atoms represent the covalent electrons between the hydrogen atoms. This line is a short version of the two dots.
A covalent bond in water:
The Lewis point structure of water shows that an electron from hydrogen and one electron from oxygen are covalently shared. The other four valence electrons inKo oxygen are paired. A line is an abbreviation for two dots that represent a covalent bond.
Lewis dots structure of compound:
As you have learned, the Lewis point structure is an easy way to graph an element and see its valence electrons. The Lewis point structure is a diagram showing the valence electrons of a part. In the Lewis point structure, the core of the element is represented by its symbol. Valence electrons are represented by dots arranged in pairs around the emblem. It makes it easy to see that the valence shell of an element is not filled. You can also write the Lewis point structure of the compound.
Summary:
For example, we know that nitrogen has five valence electrons, represented by N, and we want to fill its outer shell with a total of eight electrons.
Structural formula:
Structural formulas show the relative positions of atoms or ions in a molecule and the number and position of bonds between them. It tells us a lot about the connection. You can see the type, number, arrangement method, bonds between atoms, etc.
The procedure for describing the Lewis point structure of a compound is simple.
-
Determines the type and number of atoms in a molecule.
-
Describe the Lewis point structure for each atom.
-
Bond the atoms through electron-pair bonds so that each bit has a complete octet. If a molecule has carbon, it is always in the middle. Hydrogen is usually outside.
-
Check the work again and ensure that each atom has 8 electrons and no more.
Lewis structure drawing method:
Lewis structure outline:
Only those subatomic particles are smaller than the atom. Electrons, protons, neutrons. Even under a complex microscope, you cannot see the individual electrons surrounding the nucleus. A Lewis point structure is an image depicting the outermost shell of an electron, also known as a valence electron, and potential covalent bonds within an atom or molecule.
These valence electrons are negatively assessed and are tempted to the positively charged nuclei composed of neutrons and protons. Keep in mind that in reality, the electrons are constantly moving around the heart and are not rooted in one place, as shown in the 2D structure.
A series of points lines draw Lewis Point structure and atomic symbols to explain how atoms or molecules are placed. Lewis point structures can be created for monatomic, covalent, or polyatomic ions.
Draw the Lewis point structure using the Periodic Table.
The periodic table includes all the data required to draw the Lewis point structure. Each group or column is identified by a Roman numeral representing the number of valence electrons. It applies to the entire group. For example, every element in Group I has one valence electron in the first column.
All Group II components have two valence electrons, up to eight valence electrons. The properties are consistent across the rows or periods of the periodic table. Periods are indicated by numbers 1, 2, and 3, representing energy levels or electron shells. The first period or sequence has only one energy level to hold two electrons. Term 2 with a second shell can have two electrons.
The periodic table also shows electronegativity. The element with the highest electronegativity is in the upper right corner of the periodic table, and moving the group down or to the left of the period reduces the electronegativity. The Periodic Table provides a strong reference when dealing with electrons, covalent bonds, and polyatomic ions when drawing the Lewis point structure.
Use of Lewis point structure to represent valence electrons:
You can draw a Lewis point structure to show the valence electrons surrounding the atom itself. This type of Lewis point structure is represented by an atomic symbol and a series of points.
See the subsequent samples for how to draw Lewis point structures for common atoms that participate in covalent bonds.
Example 1: Draw the Lewis point structure of a hydrogen particle.
Hydrogen is class I, so it has one valence electron on its surface.
Example 2: Draw the Lewis point structure for the fluorine atom.
Fluorine is in period 2, so it can hold up to 8 electrons with an energy of
seconds. Fluorine belongs to group VII, which means seven valence electrons are around the atom.
Example 3: Draw the Lewis point structure for oxygen.
Oxygen is in period 2, so it can hold up to 8 electrons with an energy of seconds. The oxygen group is VI, which means there are six power electrons near the atom.
Example A: Determine the total number of power electrons in carbon:
carbon in group IV,
carbon in group 4,
total valence electron number in carbon = 4
Example B. Find the total number of valence electrons in H2O:
Group I hydrogen has 1 electron x 2 = 2
Group VI oxygen has 6 electrons. of MgBr2:
group 2 magnesium has particle 2 x 1 = 2
bromine, bunch 7 has 7 electrons x 2 = 14 Total valence electrons of
How to draw Lewis dot structure:
Step 1. Determine the total number of power electrons to represent in the Lewis diagram.
Example: Total CO2 = 16.
Step 2. Place the less electronegative element in the centre and draw single bonds from the central atom to the other bits.
Step 3. Determine how many electrons the central element needs to add. Assume that each outermost part has a full valence (2 for H, 8 for other factors) of bound and unbound electrons.
Add up all these electrons and subtract the total number of valence electrons in the molecule. CO2 has 16 valence electrons. We assume that each O has eight valence electrons. 2 × 8 = 16; 1616 = 0, so we don’t need more electrons to C.
Step 4. Add a double or triple bond to the central atom until it has a full octet.
Step 5. Add electrons to the extreme elements until they are full of bytes.
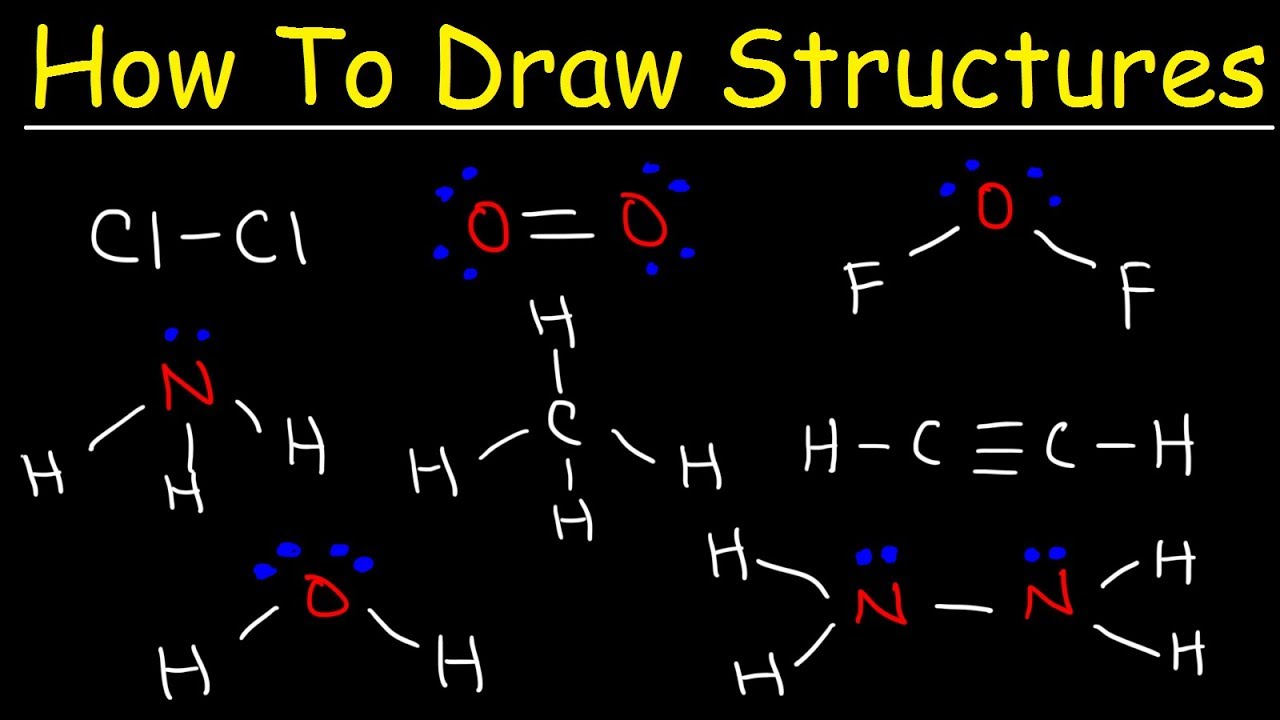
Drawing Lewis Point Structure Example for a Covalent Bond:
We will use the number of valence electrons per atom and plot them appropriately. Refer to “How to Draw Lewis Dot Structures” for step-by-step instructions.
Notice the following Lewis dot structure graphs for some covalent combinations.
Example 1. Ammonia, NH3:
Nitrogen is in class V, indicating five (5) valence electrons. There are three (3) hydrogens current, each with an individual electron of its own, making the total molecule a sum of eight (8) to consider. Since nitrogen has 5 electrons and looks for 8 to fill the second energy shell, it is satisfied by three elemental hydrogens that meet the octal rule. One unbonded nitrogen pair is left and is represented by a team of colons.
Example 2. Methane, CH4:
In this structure, non-bonding electron pairs do not exist. All of them are precisely linked into a series of lines representing two electrons each. In methane, each hydrogen has a first energy shell filled with 2 electrons, its valence electron with a shared electron from carbon, and a second energy shell of carbon-containing a total of 8 electrons, 4 electrons. private electrons and four shared electrons. (1 of each hydrogen surrounds it).
Draw Lewis point structures for polyatomic ions:
The Lewis dot structure for drawing polyatomic ions is similar to removing individual atoms or covalent compounds. However, we will be dealing with ions rather than just atoms in this case. Ions will possess a positive or negative charge, which will be reflected by the number of electrons drawn and the" or “+” sign. It means having more electrons to create a negative charge or fewer electrons to create a more positive direction.
Example 1. PO43, phosphate ion: With four oxygen present with six (6) electrons and phosphorus having five (5), there is a total of 31 electrons. However, since there is a charge of, 3mthere are a few things to consider. Phosphorus is in period 3, which means it can hold more than 8 electrons and form double bonds with oxygen, meeting the octet rule for one oxygen but not for the others.
Example 2. NH4 +, ammonium ion: We are dealing with a positively charged polyatomic ion with ammonium. The total number of valence electrons of nitrogen and four hydrogens is 9 electrons. Since there is a 1+ positive charge, there are fewer electrons, so there will be a total of 8, represented by the four bonds as a straight line.
Example 3. OH, –, hydroxide ion: How many electrons does the hydroxide ion have? Oh, oxygen has six, and hydrogen has 1. Still, since there is a negative charge on the ion, it will have an extra ion giving it a total of 8 electrons, represented by the bond pair between oxygen and hydrogen with three zeros. Bond pairs (lonely) surrounding oxygen.
Key ideas:
Specifies the complete number of power electrons in an element or compound. If the molecule consists of more than one element, add up the valence electrons of all elements present in the combination.
Lewis Point Determines which atom will be the central atom of the structure. The main atom is the smallest electronegative atom in a compound. Remember the electronegativity trend of the periodic table.
Summary:
Once identified, draw the corresponding element with the atomic symbol in the centre and single bonds on the other atoms.
Subtract the total number of valence electrons associated with the molecule by the valence electron shell of each outer atom (2 for H and 8 for all others). It distributes the remaining electrons into
unbonded pairs around the central atom, forming double and triple bonds until it has a full octet. Draws unbonded pairs around the outer atom until it includes a complete octet.
Check the work. Make sure all valence electrons and bonds are described.
Lewis Point Pattern Drawing Practice:
1. carbon
2. Sodium
3. Neon
4. Hydrochloric acid
5. H2O
6. SO2
7. NO3–
8. ClO3–
9. CN–
10. SO42
Lewis structure setup:
Creating an O2 Lewis structure is easiest to think of as dots. Oxygen must bond twice, as shown by the single dots to the left and right of the oxygen atom in the figure below. Two pairs of dots represent four more electrons that will not be bound.
Think of connecting a single point to form a bond between each O atom. Each O atom must bond twice. Thus, a pair of O atoms from two bonds with each other.
Lewis structure:
The two bonds look like two parallel lines between O atoms and are called double bonds. Each bond forms one electron pair in each bonded O atom, so two similar double bonds represent a total of four electrons.
Each O is surrounded by four dots and 2 rods or lines, representing four additional electrons in the O2 double bond.
So each O is surrounded by eight valence electrons, giving an octet and making it stable. The two letters O in the Lewis structure O2 represent the nucleus (centre) of the oxygen atom. The heart contains protons and neutrons, the solid parts of the molecule.
Summary:
Interestingly, the dots and lines represent electrons, not solids. The diagram is far off-scale because the relative size of the nucleus compared to the surrounding electrons is usually comparable to that of a pea in an arena.
Attributes:
The Lewis structure of O2 shows that the two oxygen atoms are equally bonded. It’s perfectly symmetrical.
In general, small symmetric molecules are nonpolar. The Lewis structure of O2 indicates that the O2 molecule is perfectly symmetric. Therefore, O2 is a nonpolar substance. Small nonpolar substances tend to gas. It tends to have a low boiling point.
Summary:
For example, to turn O2 into a liquid, it must be cooled to about 180°C or 300°F. The Earth doesn’t get that cold, and the atmosphere is filled with oxygen gas.
Lewis dot structure:
Lewis systems, also called Lewis dot formulas, Lewis dot systems, electron dot systems, or Lewis electron-dot systems (LEDs), are diagrams that display the bonding among atoms of a molecule and the lone pairs of electrons that could exist withinside the molecule.
A Lewis shape may be drawn for any covalently bonded molecule, in addition to coordination compounds. The Lewis shape was named after Gilbert N. Lewis, who brought it in his 1916 article The Atom and the molecule. Lewis systems enlarge the idea of the electron dot diagram by including traces among atoms to symbolize shared pairs in a chemical bond.
Lewis systems display every atom and its role within the molecule’s shape using its chemical symbol. Lines are drawn among atoms that can be bonded to 1 another (pairs of dots may be used in place of traces).
Excess electrons that shape lone pairs are represented as dots and positioned after the atoms.
Summary:
Although primary organization factors of the second one length and past normally react via way of means of gaining, losing, or sharing electrons till they have got ■■■■■■■■ a valence shell electron configuration with a complete octet of (8) electrons, hydrogen (H) can most effective shape bonds which proportion simply electrons.
Construction and electron counting:
The available quantity of electrons represented in a Lewis shape is identical to the sum of valence electrons on every man or woman atom. Non-valence electrons aren’t represented in Lewis systems.
Once the whole quantity of to be had electrons has been determined, electrons should be positioned into the shape in line with those steps:
-
The atoms are first related via means of available bonds.
-
If t is the total quantity of electrons and the number of available bonds, t-2n electrons stay positioned.
-
These need to be set as lone pairs: one pair of dots for every pair of electrons to be had. Lone pairs need to be placed on outer atoms (apart from hydrogen) until every router bit has 8 electrons in bonding pairs and lone pairs; greater lone pairs can also be positioned at the central atom. Lone pairs need to be placed on greater electronegative atoms first in doubt.
-
Once all lone pairs are positioned, atoms (mainly the central atoms) might not have an octet of electrons. In this case, the particles should shape a double bond; a lone pair of electrons is moved to shape a 2d bond among the two atoms.
As the bonding pair is shared among the two atoms, the atom that initially had the lone team nevertheless has an octet; the alternative bit now has greater electrons in its valence shell.
Lewis systems for polyatomic ions can be drawn via a similar approach. When counting electrons, bad ions need to have greater electrons positioned in their Lewis systems; effective ions need fewer electrons than an uncharged molecule.
When the Lewis shape of an ion is written, the complete body is positioned in brackets, and the price is registered as a superscript at the top right, out of doors the frames.
A less difficult approach has been proposed for the building.
Lewis systems, putting off the want for electron counting:
The atoms are drawn displaying the valence electrons; bonds are then shaped via pairing up valence electrons of the particles inside the bond-making process. Anions and cations are shaped via means including or casting off electrons to form the ideal atoms.
A trick is to matter up valence electrons, then matter up the number of electrons wanted to finish the octet rule (or with hydrogen simply 2 electrons), then take the distinction of those numbers. The solution is the number of electrons that make up the bonds. The relaxation of the electrons visit fills all of the octets of the different atoms.
Summary:
Another easy and trendy method to writing down Lewis systems and resonance bureaucracy has been proposed.
Formal Charge:
In phrases of Lewis systems, formal price is used withinside the description, comparison, and evaluation of in all likelihood topological and resonance methods via way of means of figuring out the plain digital price of every atom within, primarily based totally upon its electron dot shape, assuming exceptional covalency or nonpolar bonding.
It has made use of in figuring out feasible electron re-configuration while regarding response mechanisms and frequently outcomes withinside the equal signal because of the partial price of the atom, with exceptions.
In trendy, the formal price of an atom may be calculated using the following formula, assuming non-widespread definitions for the markup used.
The formal charge is computed because of the distinction between the number of valence electrons that a neutral bit could have and the number of electrons that belong to it within the Lewis shape.
Summary:
Electrons in covalent bonds are similarly broken up among the particles inside the cement. The general standard fees on an ion need to be identical to the price at the ion, and the whole of the typical costs on an impartial molecule need to be similar to zero.
Resonance:
For a few molecules and ions to decide which must move lone pairs to shape double or triple bonds, more unique resonance systems can be written for identical molecules or ions.
In such instances, it’s miles normal to jot down all with -manner arrows in among (see example below). It is often the case while more than one atoms of the identical kind surround the vital atom and are mainly not an unusual place for polyatomic ions. When this case occurs, the molecule’s Lewis shape is stated to be a resonance shape, and the molecule exists as a resonance hybrid.
Each of the extraordinary opportunities is superimposed at the others, and the molecule is taken into consideration to have a Lewis shape equal to a few aggregates of those states.
The nitrate ion (NO3−), for instance, need to shape a double bond between nitrogen and one of the oxygens to meet the octet power for nitrogen.
Nevertheless, because the molecule is symmetrical, it no longer depends on which oxygens paperwork the double bond. In this case, there are three viable resonance systems.
Expressing resonance while drawing Lewis systems can be accomplished both through removing every of the viable resonance paperwork and putting double-headed arrows among them or through the use of dashed traces to symbolize the partial bonds (even though the latter is a superb illustration of the resonance hybrid that is now no longer, officially speaking, a Lewis shape).
When evaluating resonance systems for the identical molecule, people with the fewest legal fees commonly contribute greater to the general resonance hybrid.
When standard prices are critical, resonance systems with bargain-basement prices are favoured at greater electronegative factors and outstanding fees at much less electronegative elements.
Single bonds can also be moved inside to create resonance systems for hypervalent molecules, including sulfur hexafluoride, which is the accurate description consistent with quantum chemical calculations rather than the not unusual place increased octet version.
Summary:
The resonance shape must now no longer be interpreted to suggest that the molecule switches among paperwork. However, the molecule acts because of the commonality of more than one piece of paperwork.
Alternative formations:
Chemical systems can be written in greater compact paperwork, mainly while displaying natural molecules. In condensed structural formulas, many or maybe all the covalent bonds can be left out, with subscripts indicating the wide variety of equal agencies connected to a selected atom.
Another shorthand structural diagram is the skeletal method (also referred to as a bond-line or carbon skeleton diagram).
Summary:
Hydrogen atoms bonded to carbon aren’t proven—they may be inferred through counting the wide variety of bonds to a selected carbon atom—every carbon is believed to have four bonds in total, so any bonds now no longer proven are, through implication, to hydrogen atoms.
Frequently Asked Questions:
Here we discuss some frequently asked questions:
Q1: What electrons do Lewis systems show?
A: The outermost crucial degree of electricity-containing electrons is known as the extent of valence and consists of electrons of valence. Lewis symbols are diagrams displaying the wide variety of valence electrons of a particular detail with dots indicating lone pairs.
Q2: What is the reason for Lewis’s systems?
A: The purpose of Lewis systems is to offer an easy manner for chemists to view molecules that lets incorrect predictions approximately the real molecules and shape and homes be made.
Q3: Is hydrogen H2 or H?
A: Hydrogen is the full, ample detail withinside the universe, and it has a wide atomic variety of one. Hydrogen has a molar mass of one, and its molecular component is H2 . Hydrogen, H, is the lightest detail with the atomic wide variety 1. It is a colourless, odourless, tasteless, and pretty flammable fuel line with the molecular components H2.
Q4: What is the Lewis Dots shape of hydrogen chloride?
A: If we desired to expose the Lewis shape of HCl, we might draw the following: We can see that the covalent bond includes electrons among the H and the Cl. The H has a complete outer shell of electrons, and the chlorine has a full outer shell of 8 electrons.
Q5: What is a molecule of hydrogen?
A: A molecule of hydrogen is the most effective feasible molecule. It includes protons and electrons held collectively with the aid of using electrostatic forces. Like atomic hydrogen, the assemblage can exist in some electricity levels.
Q6:What do the dots constitute on a Lewis dot shape?
A: Lewis dot diagrams use dots organized across the atomic image to symbolize the electrons withinside the outermost electricity degree of an atom. Single bonds are represented with the aid of using a couple of dots or one line among particles. Double bonds are represented by using pairs of dots or traces among atoms.
Q7: Why is H2 now no longer h?
A: It’s due to the fact they’re normally observed as diatomic particles. Because hydrogen atom can’t exist on its own, it is volatile, so it combines with some other hydrogen atom to shape a hydrogen molecule (H2). So the hydrogen which exists in nature freely in air is H2 now no longer H.
Q8: What are the means of H2?
A: Hydrogen fuel line.
H2, the chemical composition for the hydrogen fuel line (dihydrogen). Deuterium (Hydrogen-2, H-2, 2H) is the isotope of hydrogen with one proton, one neutron, and one electron.
Q9: How do h2 molecules shape?
A: When hydrogen atoms integrate to shape a hydrogen molecule, H2, they accomplish that in a distinctive manner from the electron switch method we were discussing. Instead of moving an electron to shape H+ and H− ions, the two atoms proportion their electrons.
Q10:What are the atoms of hydrogen?
A: The hydrogen atom is the most effective of all traces: it includes an available proton and electron. In complement to the most not uncommon place shape of the hydrogen atom, this is known as protium, different isotopes of hydrogen exist deuterium and tritium.
Conclusion:
Lewis structures also comprehended as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs), show the adhesion between particles of a molecule and the lone couples of electrons in the molecule.
READ ALSO:
https://howtodiscuss.com/t/hydrogen-peroxide-burn/115350