How to find formal charge? Formal charge is the charge which is assigned to an atom in a molecule.
Here is the formula to find the formal charge:
Formal charge= no. of valance electron - no. of dots - no. of lines
Formal charge:
Formal charge is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity.
Denotation:
-
Charge= q
-
Formal charge= F.C
Lewis formal charge structure:
When determining the best Lewis structure (or predominant resonance structure) for a molecule, the structure is chosen such that the formal charge on each of the atoms is as close to zero as possible.
Examples:

Formal charges in ozone and the nitrate anion.
-
CO2 is a neutral molecule with 16 total valence electrons. There are different ways to draw the Lewis structure.
-
Carbon single bonded to both oxygen atoms (carbon = +2, oxygen = −1 each, total formal charge = 0)
-
Carbon single bonded to one oxygen and double bonded to another (carbon = +1, oxygen double = 0, oxygen single = −1, total formal charge = 0)
-
Carbon double bonded to both oxygen atoms (carbon = 0, oxygen = 0, total formal charge = 0)
Result:
Even though all three structures gave us a total charge of zero, the final structure is the superior one because there are no charges in the molecule at all.
Pictorial method:
The following is equivalent:
• Draw a circle around the atom for which the formal charge is requested (as with carbon dioxide).
• Count up the number of electrons in the atom’s “circle.”
• Since the circle cuts the covalent bond.
• “In half,” each covalent bond counts as one electron instead of two.
• Subtract the number of electrons in the circle from the group number of the element (the Roman numeral from the older system of group numbering, NOT the IUPAC 1-18 system) to determine the formal charge.

The formal charges computed for the remaining atoms in this Lewis structure of carbon dioxide are shown below.
Summary:
It is important to keep in mind that formal charges are just that – formal, in the sense that this system is a formalism. The formal charge system is just a method to keep track of all of the valence electrons that each atom brings with it when the molecule is formed.
Formal Charge Formula:
Need to figure out if an atom is negative, positive, or neutral?
Here’s the formula for figuring out the “formal charge” of an atom:
Formal charge = [# of valence electrons] – [electrons in lone pairs + 1/2 the number of bonding electrons]
This formula explicitly spells out the relationship between the number of bonding electrons and their relationship to how many are formally “owned” by the atom.
For example, applying this to BH4 (top left corner in the image below) we get:
• The number of valence electrons for boron is 3.
• The number of non-bonded electrons is zero.
• The total number of bonding electrons around boron is 8 (full octet).
• One half of this is 4.
Result:
So formal charge = 3 – (0 + 4) = 3 – 4 = –1.
There is a slightly easier way to do this, however.
Since a chemical bond has two electrons, the “number of bonding electrons divided by 2” is by definition equal to the number of bonds surrounding the atom.
So we can instead use this shortcut formula:
Formal Charge = [# of valence electrons on atom] – [non-bonded electrons + number of bonds]
Applying this again to BH4 (top left corner)
• The number of valence electrons for boron is 3.
• The number of non-bonded electrons is zero.
• The number of bonds around boron is 4.
Result:
• So formal charge = 3 – (0 + 4) = 3 – 4 = –1.
• The formal charge of B in BH4 is negative 1.
Let’s apply it to :CH3 (one to the right from BH4)
• The number of valence electrons for carbon is 4.
• The number of non-bonded electrons is two (it has a lone pair).
• The number of bonds around carbon is 3.
Result:
• So formal charge = 4 – (2 +3) = 4 – 5 = –1.
• The formal charge of C in :CH3 is negative 1.
• Same formal charge as BH4.
Let’s do one last example
• Let’s do CH3+ (with no lone pairs on carbon).
• It’s the orange one on the bottom row.
• The number of valence electrons for carbon is 4.
• The number of non-bonded electrons is zero.
• The number of bonds around carbon is 3.
Result:
So formal charge = 4 – (0 +3) = 4 – 3 = +1. You can apply this formula to any atom you care to name.
Here is a chart for some [simple](https://howtodiscuss.com/t/simpler-times/31022) molecules along the series B C N O
| Valancey 3 | Valancey 4 | Valancey 5 | Valancey 6 | Charge |
|---|---|---|---|---|
| BH4 | :CH3 | NH2 | OH | -1 |
| BH3 | CH4 | NH3 | OH2 | 0 |
| BH2 | CH3 | NH4 | OH3 | +1 |
I hope beryllium and fluorine aren’t too offended that I skipped them, but they’re really not that interesting for the purposes of this table.
Note the interesting pattern in the geometries (highlighted in colour):
• BH4(–), CH4, and NH4(+) all have the same geometries, as do CH3(–), NH3, and OH3(+).
• Carbocation CH3(+) has the same electronic configuration (and geometry) as neutral borane, BH3. The familiar bent structure of water, H2O, is shared by the amide anion, NH2(–).
• These shared geometries are one of the interesting consequences of valence shell electron pair repulsion theory (VSEPR – pronounced “vesper“, just like “Favre” is pronounced “Farve”.)
The formal charge formula also works for double and triple bonds:
Mathematically, it can be expressed by following formula:
F.C. = [Total no. of valence e– in free state] – [total no. of e– assigned in Lewis structure]
F.C. = [Total no. of valence e– in free state] – [total no. of non-bonding pair e– (lone pair)] – 1/2 [total no. of bonding e–]
The factor of ½ is attached to the no. of bonding e– because bonding e– is shared between two atoms.
Formula Charge Calculation of SO42-:
The Lewis structure of SO42- is as follows:
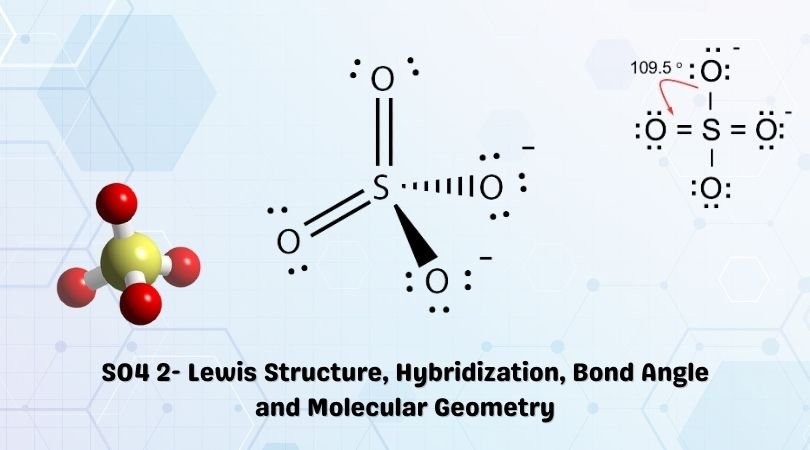
Formal Charge 1,2,3 and 4 are for the indexing of oxygen while calculating the formal charge.
| Atom | Valence e– in Free State | No. of non-bonding e– in Lewis structure | No. of bonding pairs in Lewis structure | Total no. of pairs | Formal charge |
|---|---|---|---|---|---|
| Sulphur (S) | 1 | 6 | 0 | 12 | = 6 – 0 – 12/2 =6-6 =0 |
| Oxygen (O) | – 1 | 6 | 4 | 4 | = 6 – 4 – 4/2 = 6 -6 = 0 |
| Oxygen (O) | – 2 | 6 | 6 | 2 | = 6 -6 -2/2 = 6 -7 = -1 |
| Oxygen (O) | – 3 | 6 | 4 | 4 | = 6 – 4 – 4/2 = 6 -6 = 0 |
| Oxygen (O) | – 4 | 6 | 6 | 2 | = 6 -6 -2/2 = 6 -7 = -1 |
Importance of Formal charge:
• The formal charge being a theoretical charge doesn’t indicate any real charge separation in the molecule.
• Formal charges help in the selection of the lowest energy structure from a number of possible Lewis structures for a given species.
• Knowledge of the lowest energy structure helps in predicting the major product of a reaction and also describes a lot of phenomena.
• Generally, the lowest energy structure is the one with the smallest formal charges on the atoms and the most distributed charge.
• The actual shapes of the polyatomic molecule is properly given by the number of formal charges present on any atom, molecule or ion.
• Knowing the formal charge on a particular atom in a structure is an important part of keeping track of the electrons and is important for establishing and predicting the reactivity.
• The formal charge on an atom in a molecule reflects the electron count associated with the atom compared to the isolated neutral atom.
Effect on solubility:
You want the formal charge on the elements to be as close to zero as possible. Since the formal charge can be negative or positive, the lower the absolute value of the formal charge, the higher the stability.
Zero formal charge:
The formal charge on any atom is zero when the number of protons (the atomic number) and the number of electrons that “belong” to that atom are equal. We have seen that it requires 13.6 kcal/mol to separate an electron from a hydrogen atom.
Rules:
Formal charge:
The charge assigned to an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms.
Octet rule:
Atoms lose, gain, or share electrons in order to have a full valence shell of eight electrons.
Negative formal charge:
If a formal charge of 1- is located next to a formal charge of 1+, the formal charges can usually be minimized by having a lone pair of electrons, located on the atom with the 1- charge become a bonding pair of electrons that is shared with the atom that has the 1+ formal charge this can be visualized in the same way.
Best formal charge:
The best formal charge is 0. It is best to have a formal charge of 0 for as many of the atoms in a structure as possible.
Lowest formal charges:
Lower formal charges correspond to more stability within those compounds. This is because resonance structures which have the lowest formal charge contribute the most to the Lewis structure for that compound.
Isomers formal charge:
Isomers have different arrangement of both atoms and electrons. The better ones have minimal formal charges, negative formal charges are the most electronegative atoms, and bond is maximized in the structure.
Summary:
The formal charge formula shows that which Lewis structure is the best structure. The structure which have the least formal charge or 0 charge is the best Lewis structure. By using formal charge we can calculate the electrons from the atom of molecules.
FAQ’s:
People often asks these questions:
1. What is the formal charge on Cl atom of HClO4 ion?
Formal charge on Cl atom in HClO4 ion is 3. According to the formula the formal charge can be calculated as:
F.C = Valance electron – no. of non-bonding electrons - ½(no. of bonding electrons)
F.C = 7-8/2-0
F.C = 3
2. What is the formal charge on S atom of HSO4- ion?
Formal charge on S atom in HSO4- ion is 2. According to the formula the formal charge can be calculated as:
F.C = Valance electron – no. of non-bonding electrons - ½(no. of bonding electrons)
F.C = 6-8/2-0
F.C = 2
3. How to find that which formal charge is the best?
• It is preferable to have a molecular structure with zero formal charges rather than one with some formal charges.
• If nonzero formal charges are required in the Lewis structure, the arrangement with the smallest nonzero formal charges is preferred.
4. Is formal charge is same as the charge on atom?
That is not the formal charge of an atom that has just become an ion, such as O2, but it is the net charge. The hypothetical charge for an atom when electrons are absolutely uniformly shared in a chemical bond is known as formal charge. It’s not the same as oxidation state; in fact, it’s almost the polar opposite.
5. How to find the octet rule and what are the expectations?
The octet rule, however, has three general exceptions: Molecules containing an odd number of electrons, such as NO; SF6 molecules in which one or more atoms have more than eight electrons; and. Molecules containing one or more atoms with fewer than eight electrons, such as BCl3.
6. What is the formal charge of Hydrogen?
Hydrogen atoms have only one bond and no formal charge, hence the pattern is simple. The proton, H+, and the hydride ion, H–, which is a proton plus two electrons, are exceptions to this norm.
7. What is the formal charge on NF3?
Formal charge on NF3 ion is 0. According to the formula the formal charge can be calculated as:
F.C = Valance electron – no. of non-bonding electrons - ½(no. of bonding electrons)
F.C = 7 – 0.5/2 – 6
F.C = 0
8. What is the main purpose of using formal charge?
The formal charge system is just a way of keeping track of all of the valence electrons that each atom contributes to the formation of the molecule.
9. Can we reduce the formal charge?
If a formal charge of 1- is next to a formal charge of 1+, the formal charges can usually be reduced by having a lone pair of electrons on the 1–charged atom become a bonding pair of electrons shared with the atom with the 1+ formal charge.
10. How to measure the negative formal charge?
If an atom provides fewer electrons than usual while maintaining an octet, it must be acquiring extra electrons from somewhere else. It will be formalized with a negative charge. If the atom does not have the standard amount of bonds, formal charge is often present.
11. Why is formal charge used for?
The formal charge system is just a method to keep track of all of the valence electrons that each atom brings with it when the molecule is formed.
12. What is the formal charge of Carbon dioxide?
CO2 is a neutral molecule with 16 total valence electrons. Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge =0). Since carbon has 4 valence electrons, its formal charge will be zero.
13. Is the formal charge considered as an actual charge?
Formal charges are not real charges, they are a way of looking at electron distributions in a Lewis dot structure.
14. Why common Oxygen has zero formal charge?
The common arrangement of oxygen that has a formal charge of zero is when the oxygen atom has 2 bonds and 2 lone pairs.
15. Define formal charge and give its examples?
A formal charge is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. Example: The formal charge of CO2 is zero.
16. What formal charge mentions its formula?
Formal Charge = Valence electrons – No. of non-bonding electrons – ½ (No. of bonding electrons).
17. What are the rules of formal charge?
The formal charge of the atom, the sum of the charge of the proton and the charge of the electron, is zero. The formal charge on any atom is zero when the number of protons (the atomic number) and the number of electrons that “belong” to that atom are equal.
18. Why is formal charge important?
Formal charge on a particular atom in a structure is an important part of keeping track of the electrons and is important for establishing and predicting the reactivity.
19. What does formal charge represent?
The formal charge of an atom in a molecule is the charge that would reside on the atom if all of the bonding electrons were shared equally.
20. How does the formal charge affect the solubility of an atom?
Formal charge is inversely related with the solubility of an atom. A formal charge can be negative or positive, the lower the absolute value of the formal charge, the higher the stability.
Conclusion:
Formal charge is required for showing a complete and correct Lewis-Kekulé structure in organic chemistry, according to convention. Inorganic chemistry is different in this case. The most excellent structure variation is that of a molecule with the least amount of charge. The valence bond theory of Slater and the molecular orbital theory of Mulliken were developed as a result of the disparities between formal charge and oxidation state. We can find the formal charge by knowing the Lewis structure and number of lone and bond pairs in the atom. The formal charge at its lowest position is best.


