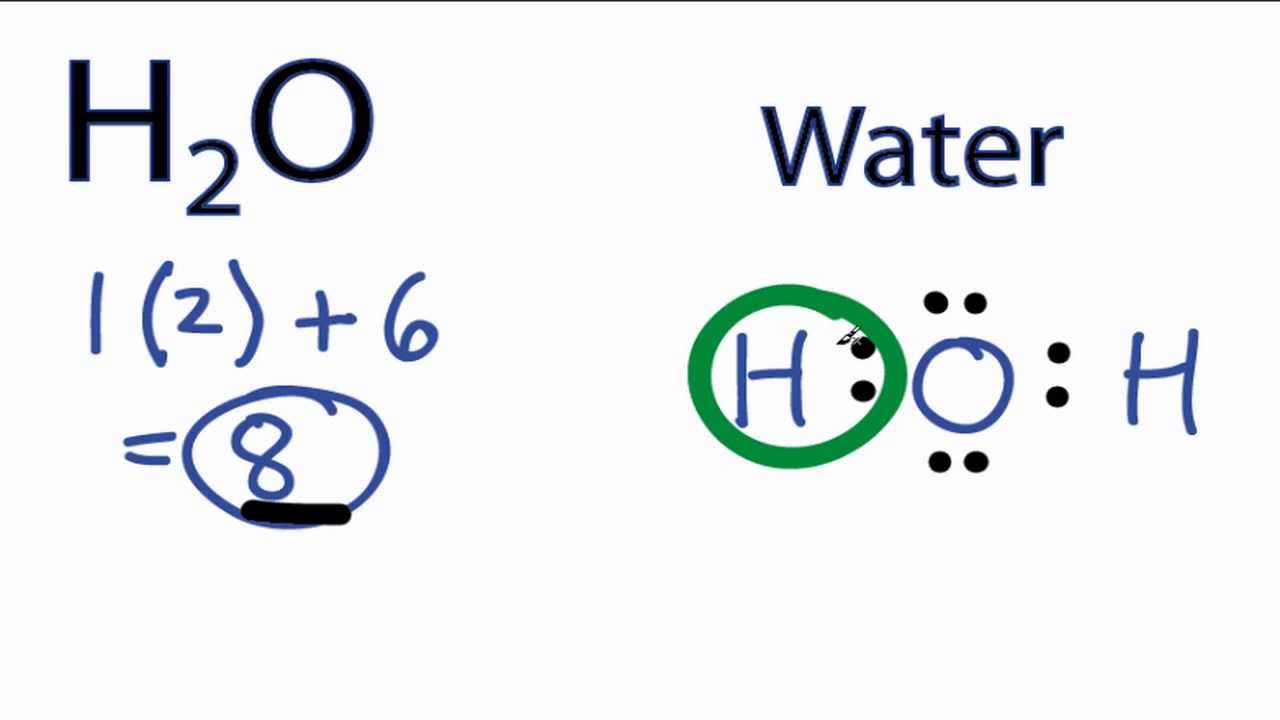H2O Lewis Structure exhibits two solitary sigma bonds. These connections leave two lone electron pairs on the oxygen atom, contributing to the H2O molecule’s tetrahedral bent geometry.
Lewis Structure of H2O
One of Earth’s most abundant elements, water (with the chemical formula H2O), is essential to life on our planet. Two hydrogens and one oxygen atom are covalently linked to form a single molecule. A compound is formed when two or more H2O molecules join via hydrogen bonds.
Lewis structures, also known as electron dot structures, are used to graphically illustrate the number of valence electrons in an atom, which is available to participate in bond formation and lead to the synthesis of molecules and compounds.
Note: It’s fascinating to consider that water rapidly interacts with most of the elements in the periodic table because covalent bonds are stronger than hydrogen bonds.
Drawing the Lewis Structure of Water: Step-by-Step Instructions
In this guide, you’ll learn all about the lewis structure of an H2O molecule and how to create it. Some of these procedures are rarely employed since the water molecule is so easy to work with. When this occurs, they are highlighted along with the necessary procedures.
-
Discover the sum of the electrons in the valence shells of hydrogen and oxygen atoms.
-
Quantity of bonded and unpaired electrons
-
Atomic lone pairs are shown by selecting the center atom.
-
Notate the presence of atomic charges.
-
The optimum lewis structure may be achieved by checking the stability and reducing atom charges by forming bonds between lone pairs.
Important Points To Understand H2O Lewis Structure
It is necessary to clarify several fundamental principles like valence electrons, the octet rule, and the formal charge before drawing the lewis structure of water.
| Points | Explanation |
|---|---|
| Valence Electrons and the Valence Shell | The number of electrons in an element’s outermost shell is directly related to its valency. |
| Octet Rule | Atoms of other elements prefer to fill their outermost shell with eight electrons. This is known as the octet rule. |
| Formal Charge | The formal charge of the element is a quantity that shows whether the atom in question is electrostatically balanced or imbalanced. |
Summary
As a result, valence electrons are associated with the electrons in the outermost shell of an atom, which is why both terms are used interchangeably. An electrostatic equilibrium exists between the O atoms in water. O in water has no formal charge; hence the water is neutral.
Orbital Diagram for Water Molecules (H2O)
The chemical bonding between molecules in a substance can be seen using a picture called a molecular orbital diagram.
-
The molecular orbital diagram is also useful for seeing the impact of lone pairs and how two sigma bonds came to be.
-
It is clear from the preceding figure that the six valence electrons are forming covalent bonds with the hydrogen atom’s 1s orbital electrons.
-
Atomic orbitals of comparable energies blend and overlap with one another. It’s happening so that the lower-energy bonding electrons combine into higher-energy antibonding molecular orbitals.
-
Due to electron shortage, the left oxygen electrons can no longer overlap. When compared to hydrogen, oxygen has a greater electronegativity.
Because of this, oxygen has a more negative charge than hydrogen. Due to this, oxygen can draw in adjacent electrons and form a connection.
Summary
However, the hydrogen does not react with neighboring molecules since it has completed its orbital and formed a strong sigma bond with the oxygen. Even though water has no net charge, it causes polarity in an H2O molecule. There’s also an engaging piece about water polarity that you might want to read.
Hybridization of H2O molecule
In the water molecule, the bonds between the oxygen and hydrogen atoms are all sigma, and there are no pi bonds. The strongest covalent bonds are sigma bonds, as is well known.
Therefore, the bond between oxygen and hydrogen is quite stable. The two lone pairs on an oxygen atom create a significant difference. A water (H2O) molecule exhibits sp3 hybridization, meaning its oxygen is hybridized.
The diagram shows that one oxygen atom in an H2O molecule contains one 2s orbital and three 2p orbitals. These four cause the production of four sp3 hybridized orbitals as a group.
Summary
As a result, the tetrahedral bent geometry of water is formed, with s features accounting for about 25% of the molecule and p characteristics accounting for about 75%. Hydrogen-oxygen-water (H2O) molecular orbital diagrams help provide more explanation.
Properties of H2O
Quick Information
| Element | H2O |
|---|---|
| Boiling point: | 100 °C |
| Formula: | H₂O |
| Melting point: | 0 °C |
| Density: | 997 kg/m³ |
| Molar mass: | 18.01528 g/mol |
The chemical formula for water, H2O, denotes a compound of two hydrogens and one oxygen atom. It is organized in a straightforward basic fashion. In chemistry, the presence of the water component may be observed in various chemical processes due to water’s universal solvent quality.
-
Cohesion and stickiness are properties that water possesses. This is due to the collective activity of hydrogen bonding between the water molecules, which gives it its cohesion property. In addition to that, due to its polar nature, it possesses adhesion qualities.
-
At a pressure of one atmosphere, the temperature at which water begins to boil is 100 degrees Celsius, and the temperature at which water begins to melt is 0 degrees Celsius.
-
One type of polar inorganic compound is water.
-
Because hydrogen bonds between water molecules are so strong, water has an extraordinarily high surface tension for a substance with a temperature of 25 degrees Celsius, which is 71.99 mN/m.
Summary
When compared to other liquids, water is considered a “universal solvent” due to its capacity to dissolve a greater variety of things, but not all of them. Water is a colorless, odorless, and flavorless liquid with no taste.
Frequently Asked Questions - FAQs
Some related questions are given below:
1 - What is the three-dimensional structure of the H2O molecule?
According to the VSEPR hypothesis, the existence of repulsions caused by lone pair-lone pair interactions, lone pair-bond pair interactions, and bond pair-bond pair interactions leads to the water molecule taking the form of a bent V. Therefore; the correct answer is a V-shape.
2 - Why does water form tetrahedral crystals?
The melting of hexagonal ice results in a slight increase in the coordination number of liquid water, defined as the area under the first peak of the oxygen–oxygen radial distribution function, gOO(r). This causes liquid water to be classified as tetrahedral, the most common type of liquid.
3 - What kind of structure does water have?
One oxygen atom in water is connected to two separate hydrogen atoms. This makes water a very simple molecule. Polar covalent connections have formed between the atoms due to the oxygen atom’s increased electronegativity (polar bonds).
4 - Is water a tetrahedral or bent molecule?
The molecule of H2O is curved because it is a polar molecule, which means that the dipoles in the molecule do not cancel each other out. This is because the oxygen atom contains lone pairs responsible for causing repulsion and giving the atom its bent form. The fact that it possesses bond angles of 104.5 degrees disqualifies it as a tetrahedral structure.
5 - Is H2O trigonal planar?
Two lone pairs of oxygen are found in water molecules. The VSEPR hypothesis states that there are repulsions between lone pairs, and because of these repulsions, the water molecule tends to adopt a bent form, sometimes known as a V-shape.
6 - Why is water curved rather than linear?
Explanation: The electrons that make up the core oxygen atom are arranged in four pairs. There is a single covalent link between hydrogen atoms and two of the shared pairs. The other two pairings are unique and do not belong to the other atoms (non-bonding pair).
7 - Why doesn’t water flow in a straight line?
The oxygen atom in water is made up of two different lone pairs. Because of the strong repulsion between these two lone pairs and the hydrogen-oxygen-linked pairs, the molecule’s energy level is at its lowest when the angle between the hydrogen-oxygen bonds is 104.5 degrees. As a consequence of this, the water molecule is an example of a non-linear system.
8 - Why does H2O have a bent structure instead of a tetrahedral one?
Due to the proximity of the lone pairs to the oxygen atom, the H-O-H angle in water tends to be constrained to be lower than the ideal tetrahedral geometry of 109.5 degrees. As a result, water is a bent molecule with an H-O-H angle of 105 degrees.
9 - How can I draw the H2O Lewis Structure?
As H20 Lewis Structure is very popular among high school and college students.
10 - Does the structure of water have a tetrahedral shape?
Around the oxygen atom at the center of water’s molecule, there are four distinct zones of electron density (2 bonds and 2 lone pairs). These are laid out in a pattern that resembles a tetrahedron. The resultant conformation of the molecule is curved, with an H-O-H angle of 104.5 degrees.
Conclusion
In the Lewis structure of the triatomic molecule H2O, there are two single sigma bonds between the atom of oxygen and each of the hydrogen atoms. In addition, these connections leave two lone pairs of electrons on the oxygen atom, which is the primary factor that leads to the tetrahedral bent geometrical structure of the H2O molecule.
Because of this, the bond angle, which ought to be 109.5 degrees, is 104.5 degrees. Because the H2O molecule has one s orbital, and three p orbitals, which combine to generate four hybrid orbitals, the hybridization of this molecule is referred to as sp3.