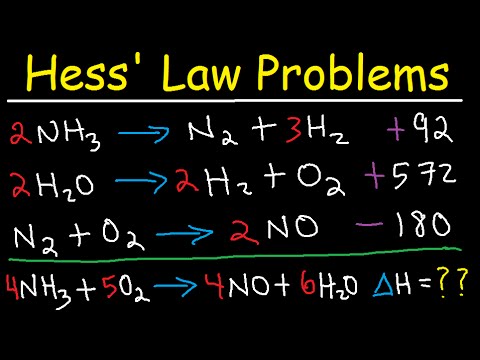
Hess’s law , also called Hess’s law of constant heat summation or Hess’s law of heat summation , rule first enunciated by Germain Henri Hess, a Swiss-born Russian chemist, in 1840, stating that the heat absorbed or evolved (or the change in enthalpy) in any chemical reaction is a fixed quantity and is independent of the path of the reaction or the number of steps taken to obtain the reaction.
 What is Hess Law?
What is Hess Law?
According to Hess’ law, also known as Hess’ law of constant heat summation, “under constant temperature, heat energy changes (enthalpy - Hrec) during a chemical reaction will stay constant, independent of the manner the reactants react to generate product.”
The state function character of enthalpy and the first law of thermodynamics are the foundations of Hess’ law. A state function is the energy (enthalpy) of a system (molecule). As a result, the enthalpy of reactant and product molecules remains constant regardless of their origin or creation path.
The first law of thermodynamics asserts that before and after any (physical or chemical) change, the total energy of the components should be equal. The total energy of the reactant must equal the total energy of the product, according to the law.
Any energy difference between the reactants and products is also fixed at a certain temperature and does not fluctuate with the reactants’ path to generate products. As a result, heat energy might be included in the process as a reactant or product of the reaction.
Hence, exothermic reactions can be written as: A + B → C + D + ΔH
Similarly, endothermic reactions become: A + B + ΔH → C + D
This enables the treatment of reactions comprising reactants and products as algebraic equations and the application of mathematical procedures to them. It’s important to keep in mind that an exothermic reaction in one direction will be endothermic in the other, and vice versa.
 Importance of Hess Law
Importance of Hess Law
Energy exists in every substance (atom/molecule). Internal energy is determined by the type of force present in the substance as well as the temperature. When a substance goes through chemical reactions, some bonds between atoms are broken and others are formed. The breaking and formation of bonds both require energy.
As a result, product chemicals in reactions may have less, equal, or greater energy than responding substances.
As a result, reactions can either release heat and become exothermic or absorb heat and become endothermic. Reactants may react further to produce the product in one of two ways:
-
In a single step or
-
In a multi-step process.
-
In addition to other products.
Understanding the energy changes in each reaction is essential for controlling the reactants and products in a chemical process to satisfy our requirements.
Internal energy change E denotes heat energy changes measured at constant volume, while enthalpy change H denotes heat energy changes measured at constant pressure.
Only the net value of all reactions or products generated is determined by the experimental data. An intermediate reaction step’s enthalpy change, or any intermediate product’s enthalpy change, cannot be measured experimentally.
In excess oxygen, carbon, for example, interacts with oxygen to generate carbon dioxide. Carbon and oxygen react directly to produce carbon dioxide, or in two steps to produce carbon monoxide and then carbon dioxide. The energy changes for the generation of carbon dioxide will be measured, but not for carbon monoxide.
Similarly, calculating the enthalpy of creation of benzene from carbon and hydrogen is impossible since in the given conditions, carbon and hydrogen can combine to generate not only benzene but also other forms of hydrocarbons.
Hess’s law is useful for determining non-measurable enthalpy changes in physical and chemical transformations, and it is the sole way to do so.
 Forms of Hess Law
Forms of Hess Law
 Multi-step reactions:
Multi-step reactions:
The total of all the reactants, products, and accompanying energy changes will give the reactant, products, and heat energy changes of the overall reaction if reactants react to generate products not in a single step but in a series of steps including multiple intermediary products. Heat energy changes, like molecules, can be subjected to mathematical processes.
 Multi-different reactions:
Multi-different reactions:
If the starting materials of a required chemical reaction can be acquired by adding the enthalpies of many other chemical reactions, then the enthalpy of the required reaction of reactants to products may also be found by adding the enthalpies of all those chemical processes.
 A: Hess law and multi-step reaction:
A: Hess law and multi-step reaction:
Reactant can create product B in three different ways. In the other stepwise processes, C, D, and E are intermediate. The enthalpy of the reaction (H1) is the same regardless of the path, according to Hess’ law.
As a result, the enthalpy of the direct single-step reaction and the alternative routes that lead to intermediates C, D, and E should be the same. H1 = H2 + H3 = H4 + H5 + H6 = H4 + H5 + H6.
Example: In a single step, carbon interacts with oxygen to generate carbon dioxide, producing 94.3kcals of heat. Carbon can also react in a two-step process to produce carbon mono-oxide, which is then transformed back to carbon dioxide. (H = released heat)
C + O2 → CO + 26.0 kcals
CO + O2 → CO2 + 68.3kcals
On adding the two reactions, C + O2 → CO2 + 94.3kcals
As per Hess law, ΔH = ΔH1 + ΔH2 = -26.0 + 68.3 = 94.3kcals
Both reactions have the same net reaction enthalpy as single-step formation. As a result, the enthalpy of a reaction does not change depending on the path taken by the reactants.
Summary:
Energy exists in every substance (atom/molecule). Internal energy is determined by the type of force present in the substance as well as the temperature. Reactants and products in chemical processes can release heat and become exothermic or absorb heat, and become endothermic. Hess’s law is useful for determining non-measurable enthalpy changes in physical and chemical transformations.
 B: Hess law and multi-different reactions:
B: Hess law and multi-different reactions:
Carbon, sulphur, and carbon disulphide combustion are exothermic, with enthalpies of - 393.5kJ, -296.8kJ, and -1075kJ, respectively.
Reactions are-
C(s) + O2 (g) → CO2 (g) + 393.5 kJ ………(1)
S(s) + O2 (g) → SO2 (g) + 296.8 kJ .…….(2)
CS2 (l) + 3O2(g) → CO2(g) + 2SO2(g) + 1075.0 kJ ……….(3)
Even without completing experiments, these reactions and enthalpy changes can be considered as algebraic equations to obtain the heat of production of carbon disulphide.
Equation 1: C(s) + O2 (g) → CO2 (g) + 393.5 kJ
2x equation 2:2S(s) + 2O2 (g) → 2SO2 (g) + 593.6 kJ
Reverse of equation3: CO2(g) + 2SO2(g) → CS2 (l) + 3O2(g) -1075.0 kJ
Adding the three reactions: C (s) + 2S (s) → CS2 (l) -87.9 kJ
Carbon disulphide formation is an endothermic process.
 Application of Hess law of Heat Summation
Application of Hess law of Heat Summation
Heat changes that cannot be detected experimentally can be estimated using Hess’ equation of heat summation.
 1. Enthalpy change in a physical change
1. Enthalpy change in a physical change
Carbon has two allotropes: carbon and diamond. However, because the process cannot be carried out, the energy change in the conversion of graphite to diamond cannot be established. Even yet, using Hess law, the heat changes for this hypothetical physical change may be estimated. The heat of reaction for graphite and diamond combining with oxygen is -393.4kJ and – 395.4kJ, respectively.
C (graphite) + O2 → CO2 ΔHgr = -393.4kJ
C (diamond) + O2 → CO2 ΔHdi = -395.4kJ
Reversing the diamond combustion reaction as:
CO2 → C (diamond) + O2 ΔHdi = + 395.4kJ
Adding,
C (graphite) + O2 → CO 2 ΔHgr = – 393.4kJ
Htr = +2.kJ C (graphite) C (diamond)
The allotrope conversion of graphite to diamond is 2KJ endothermic in terms of enthalpy change.
 2. Enthalpy change of chemical reaction
2. Enthalpy change of chemical reaction
Hydrogen, iodine, and hydrogen iodide have binding energies of 218 kJ, 107 kJ, and 299 kJ, respectively.
Calculate the enthalpy of forming hydrogen iodide. Is this an endothermic or exothermic reaction?
The reaction-induced formation of hydrogen iodide from hydrogen and iodine follows the reaction-induced formation of hydrogen iodide from hydrogen and iodine.
frac{1}{2}21H2 (g) + \frac{1}{2}21I2 (g) → HI (g)
The heat changes that occur when one atom of hydrogen and one atom of iodine react to generate one mole of hydrogen iodide in standard conditions are known as the enthalpy of formation of hydrogen iodide (as gas). A molecular bond must be broken to obtain one atom of hydrogen or iodine.
Bond energy of HI – Bond dissociation of H2 – Bond dissociation energy of I2 = Heat of formation
299 minus (218 + 107) = 299-325 = -26kJ
The reaction is exothermic because the heat of creation is negative.
 3. Enthalpy of formation
3. Enthalpy of formation
Many hydrocarbons can be created when carbon and hydrogen meet. As a result, the heat of benzene formation cannot be established experimentally. Hess law can be used to determine the heat change.
6C + 3H2 → C6H6 ΔH C6H6 = ?
Carbon dioxide and water have heats of production of -393.5kJ and -285.8kJ, respectively. Benzene has a heat of combustion of -3301kJ.
C + O2 → CO2 ΔH1 = -393.5kJ……1
H2 + O2 → H2 O ΔH2 = -285.8kJ……2
C6H6 + 9O2 → 6CO2 + 3H2 O ΔH3 = -3301kJ …….3
6 x Reaction 1: 6C + 6O2 6CO2 6H1 = -2361kJ… 6 x Reaction 2: 6C + 6O2 6CO2 6H1 = -2361kJ… 1
3H2 + 3O2 3H2 O 3H2 = -857.4kJ… 3 x Reaction2: 3H2 + 3O2 3H2 O 3H2 = -857.4kJ…2
Reverse of reaction 3: 6CO2 + 3H2O → C6H6 + 9O2 -ΔH3 = +3301kJ …….3
Adding the three reactions- 6C + 3H2 → C6H6 ΔH= +82.6kJ
Heat of formation of benzene is 82.6kJ.
Summary:
Carbon disulphide formation is an exothermic process, with enthalpy changes of - 393.5kJ, -296.8kJ and -1075kJ. The heat of reaction for graphite and diamond combining with oxygen can be estimated using Hess’ equation of heat summation. Calculate the enthalpy of forming hydrogen iodide. A molecular bond must be broken to obtain one atom of hydrogen or iodine.
 Derivation of Hess’s Law
Derivation of Hess’s Law
Hess’s law is a physical chemistry relationship named after Germain Hess, a Swiss-born Russian chemist and physician.If a reaction occurs in multiple steps, this law asserts that the standard reaction enthalpy for the overall reaction equals the sum of the standard enthalpies of the intermediate reaction steps, providing each step occurs at the same temperature.
Hess’s law is derived directly from the first law of thermodynamics, which is embodied in the law of energy conservation. Because enthalpy is a state function, the change in enthalpy between products and reactants in a chemical system is independent of the path taken from the beginning to the final state of the system.
When a chemical process can be broken down into multiple intermediate phases that are easier to characterise individually, Hess’s law can be used to calculate the total energy required. The enthalpy change in exothermic processes is negative, whereas the enthalpy change in endothermic reactions is positive.
C(s){graphite}→C(s){diamond}ΔHrxn=?C(s){graphite}→C(s){diamond}ΔHrxn=?
Turning graphite into diamond necessitates extremely high temperatures and pressures, making it impractical to do in a lab. This reaction’s change in enthalpy cannot be determined empirically. However, because we know the typical enthalpy change for these two compounds during oxidation, we can use Hess’s rule to determine the enthalpy change for this reaction. The following are our intermediate steps:
C(s){graphite}+O2(g)→CO2(g)ΔH∘=−393.41kJ/molC(s)
{graphite}+O2(g)→CO2(g)ΔH∘=−393.41kJ/mol
C(s){diamond}+O2(g)→CO2(g)ΔH∘=−395.40kJ/molC(s)
{diamond}+O2(g)→CO2(g)ΔH∘=−395.40kJ/mol
We must reverse the second step in order for these intermediary reactions to contribute to our net total reaction. Keep in mind that when utilising Hess’s rule to reverse reactions, the sign of H will change.
You may need to multiply a specified reaction intermediate by an integer on occasion. You must always multiply your H value by that same integer in such circumstances. We get: by rewriting the first equation and flipping the second equation:
C(s){graphite}+O2(g)→CO2(g)ΔH∘=−393.41kJ/molC(s)
{graphite}+O2(g)→CO2(g)ΔH∘=−393.41kJ/mol
CO2(g)→C(s){diamond}+O2(g)ΔH∘=+395.40kJ/molCO2(g)→C(s)
{diamond}+O2(g)ΔH∘=+395.40kJ/mol
Carbon dioxides and oxygens cancel out when these equations are added together, leaving simply our net equation. We may add the H values for these intermediary reactions using Hess’s rule to reach our final value, HrxnHrxn.
C(s){graphite}→C(s){diamond}ΔH∘rxn=1.89kJ/molC(s){graphite}→C(s){diamond}ΔHrxn∘=1.89kJ/mol
 Hess’s law lesson
Hess’s law lesson
To find the heat of reaction for a particular reaction, this course employs two methods. It begins by discussing how to combine reactions according to Hess’s law and their heats of reaction, and then moves on to discussing how to get the overall heat of reaction using standard temps of formation of the reactants and products.
 Frequently Asked Questions:
Frequently Asked Questions:
1: What is Hess law explain?
According to Hess’s law, the total energy change in a chemical reaction is equal to the sum of the energy changes in the individual reactions that make up the reaction. The law is based on the first rule of thermodynamics and energy conservation.
2: What is Hess law class 11?
The overall enthalpy change throughout a full chemical reaction is the same regardless of the path taken by the chemical reaction, according to Hess’s law. Hess’s law can be thought of as an implementation of the idea of energy conservation.
3: What is Hess law example?
The enthalpy of the fuel has been boosted by converting methanol to formaldehyde and hydrogen. When formaldehyde and hydrogen are burned together, they produce 86 kJ more energy than methonol! H stands for per mole, which implies that each mole of methanol burned releases 677 kJ of heat.
4: What is Hess law BYJU’s?
"At constant temperature, heat energy changes (enthalpy - Hrec) following a chemical reaction will stay constant, independent of the way the reactants react to produce product," according to Hess’ law, also known as Hess’ law of constant heat summation.
5: What is Hess law of constant heat summation with example?
The overall enthalpy change during a reaction is the same whether the reaction takes place in one step or multiple steps, according to Hess’s law of heat summation. www.docbrown.info. For example, in the picture above, H1=H2+H3=H4+H5+H6.
6: What do you mean by thermodynamic?
The study of the relationships between heat, work, temperature, and energy is known as thermodynamics. The rules of thermodynamics define how energy evolves in a system and whether it can do beneficial work on its surroundings.
7: What is the importance of Hess law of constant heat summation?
Hess’s Law of Constant Heat Summation, or simply Hess’s Law, states that even if a reaction has numerous steps and stages, the total enthalpy change will be the sum of all changes. This law demonstrates that the quantity enthalpy is a state function.
8: Why do we use Hess law?
Hess’s law can be used to calculate the total energy necessary for a chemical reaction that can be broken down into smaller synthetic steps that are easier to characterise independently. This allows for the collection of standard formation enthalpies, which can be used to forecast enthalpy change in complex syntheses.
9: What is Hess Law in thermochemistry?
Hess’s law, also known as Hess’s law of constant heat summation or Hess’s law of heat summation, is a rule first proposed by Germain Henri Hess, a Swiss-born Russian chemist, in 1840. It states that the heat absorbed or evolved (or the change in enthalpy) in any chemical reaction is a fixed quantity that is independent of the rate of reaction.
10: What is entropy in chemistry?
Entropy is a measure of a system’s unpredictability or chaos. The entropy increases as the unpredictability increases. The entropy of the solid state is the lowest, the entropy of the gaseous state is the highest, and the entropy of the liquid state is in the middle. The entropy change is the change in its value during a process.
Conclusion:
Hess’s law asserts that the standard reaction enthalpy for the overall reaction equals the sum of the standard enthalpies of the intermediate reaction steps. Hess’s law is derived directly from the first law of thermodynamics, embodied in the law of energy conservation.
Related Articles:
https://howtodiscuss.com/t/forces-acting-on-a-soccer-ball-when-kicked/73636?u=awais_nasir
https://howtodiscuss.com/t/wrights-law/147592?u=awais_nasir