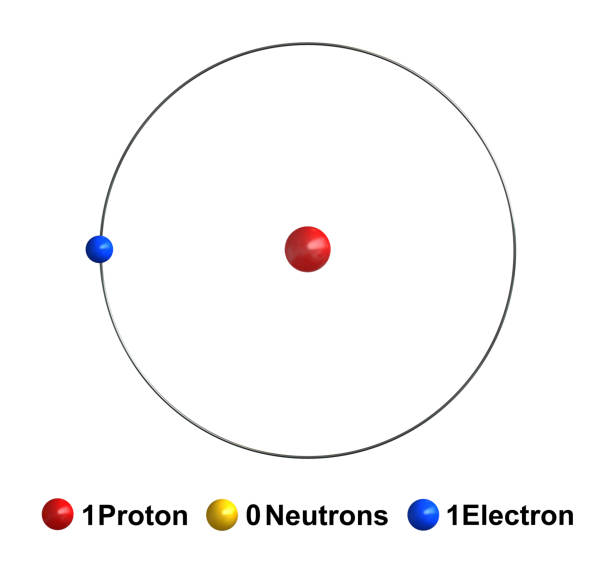
How many neutrons in hydrogen? Hydrogen has no neutrons, while deuterium has one and tritium has two. The mass numbers of hydrogen isotopes are one, two, and three, respectively. As a result, their nuclear symbols are 1H, 2H, and 3H. These isotopes’ atoms have one electron to balance the charge of the single proton.
The Isotopes of Hydrogen
![]() It is frequently beneficial to understand the simplest system. As a result, hydrogen, the most basic nucleus, has received a great deal of attention. Many of the phenomena seen in more sophisticated nuclei can be seen in hydrogen isotopes. (An isotope is a nucleus with the same Z but a different A.)
It is frequently beneficial to understand the simplest system. As a result, hydrogen, the most basic nucleus, has received a great deal of attention. Many of the phenomena seen in more sophisticated nuclei can be seen in hydrogen isotopes. (An isotope is a nucleus with the same Z but a different A.)
![]() The element hydrogen has three isotopes: hydrogen, deuterium, and tritium. How do we tell the difference? They each have one proton (Z = 1) but differ in the number of neutrons they have.
The element hydrogen has three isotopes: hydrogen, deuterium, and tritium. How do we tell the difference? They each have one proton (Z = 1) but differ in the number of neutrons they have.
![]() Energy can be emitted as a photon, which is a packet of electromagnetic radiation. Gamma rays (denoted by the Greek letter gamma, g.) are photons produced by nuclear processes. When a proton and a neutron combine to make deuterium, the process is represented as 1n + 1H 2H + g. In this equation, energy must be balanced.
Energy can be emitted as a photon, which is a packet of electromagnetic radiation. Gamma rays (denoted by the Greek letter gamma, g.) are photons produced by nuclear processes. When a proton and a neutron combine to make deuterium, the process is represented as 1n + 1H 2H + g. In this equation, energy must be balanced.
![]() Mass can be expressed in atomic mass units (u) or in million electron-volts divided by the square of the speed of light (MeV)/c2. (E = mc2, u = 931.5 MeV/c2 from Einstein’s mass-energy equivalency equation.)
Mass can be expressed in atomic mass units (u) or in million electron-volts divided by the square of the speed of light (MeV)/c2. (E = mc2, u = 931.5 MeV/c2 from Einstein’s mass-energy equivalency equation.)
![]() The deuterium nucleus has a mass of 2.01355 u, which is less than the sum of the masses of the proton (1.00728 u) and the neutron (1.00866 u), which is 2.01594 u. What happened to the lost mass (0.00239 u)? The attractive nuclear interaction between nucleons has created a negative nuclear potential energy–the binding energy EB–which is connected to the missing mass, D m. (the difference between the two masses).
The deuterium nucleus has a mass of 2.01355 u, which is less than the sum of the masses of the proton (1.00728 u) and the neutron (1.00866 u), which is 2.01594 u. What happened to the lost mass (0.00239 u)? The attractive nuclear interaction between nucleons has created a negative nuclear potential energy–the binding energy EB–which is connected to the missing mass, D m. (the difference between the two masses).
![]() The photon emitted during the deuterium formation process has an energy of 2.225 MeV, which is equivalent to the 0.00239 u necessary to dissociate the proton and neutron back into unbound particles. Nuclear decay photons have higher energy than photons produced by atomic processes.
The photon emitted during the deuterium formation process has an energy of 2.225 MeV, which is equivalent to the 0.00239 u necessary to dissociate the proton and neutron back into unbound particles. Nuclear decay photons have higher energy than photons produced by atomic processes.
![]() When a neutron is added to deuterium to generate tritium, 1n + 2H 3H+ g, a greater amount of energy is released–6.2504 MeV. The higher binding energy of tritium compared to deuterium demonstrates that the nuclear potential energy does not increase in a linear fashion with the addition of nucleons (the total binding energy is roughly proportional to A).
When a neutron is added to deuterium to generate tritium, 1n + 2H 3H+ g, a greater amount of energy is released–6.2504 MeV. The higher binding energy of tritium compared to deuterium demonstrates that the nuclear potential energy does not increase in a linear fashion with the addition of nucleons (the total binding energy is roughly proportional to A).
![]() The binding energy per nucleon increases as protons and neutrons are added to form increasingly massive nuclei, reaching a peak of about 8 MeV per nucleon around A = 60, after which the average binding energy per nucleon gradually reduces up to the most massive nuclei, where it is about 7 MeV.
The binding energy per nucleon increases as protons and neutrons are added to form increasingly massive nuclei, reaching a peak of about 8 MeV per nucleon around A = 60, after which the average binding energy per nucleon gradually reduces up to the most massive nuclei, where it is about 7 MeV.
![]() How can a nucleus, which can contain up to 100 protons, keep itself together? Why doesn’t the electrical repulsion between all those positive charges force the nucleus to disintegrate? There must be a strong enough attractive force to overcome the repulsive Coulomb forces between protons.
How can a nucleus, which can contain up to 100 protons, keep itself together? Why doesn’t the electrical repulsion between all those positive charges force the nucleus to disintegrate? There must be a strong enough attractive force to overcome the repulsive Coulomb forces between protons.
![]() Experiment and theory have revealed an enticing nuclear interaction that occurs when nucleons are close enough together (when the range is short enough). The balance of electromagnetic and nuclear forces determines the maximum size of a nucleus.
Experiment and theory have revealed an enticing nuclear interaction that occurs when nucleons are close enough together (when the range is short enough). The balance of electromagnetic and nuclear forces determines the maximum size of a nucleus.
| Isotopes of Hydrogen | Number of Protons | Number of Neutrons | Atomic Mass | Symbol |
|---|---|---|---|---|
| Protium or Hydrogen-1 | 1 | 0 | 1.00782504 or 1 | HH or 1H |
| Deuterium or Hydrogen-2 | 1 | 1 | 2.01410178 or 2 | {H or 2H |
| Tritium or Hydrogen-3 | 1 | 2 | 3.0160492 or 3 | }H or 3H |
Summary
Many of the behaviours observed in more complex nuclei can also be observed in hydrogen isotopes. When a proton and a neutron combine to form deuterium, the reaction is denoted as 1n + 1H 2H + g. A photon, which is a packet of electromagnetic radiation, can be used to emit energy.
Properties of Isotopes of Hydrogen
![]() There are three naturally occurring isotopes of hydrogen: 1H (protium), 2H (deuterium), and 3H (tritium). Other highly unstable nuclei (4H to 7H) have been created in the laboratory but do not exist in nature.
There are three naturally occurring isotopes of hydrogen: 1H (protium), 2H (deuterium), and 3H (tritium). Other highly unstable nuclei (4H to 7H) have been created in the laboratory but do not exist in nature.
![]() Tritium is the most stable radioisotope of hydrogen, with a half-life of 12.32 years. All heavier isotopes are synthetic with half-lives smaller than a zeptosecond (10-21 sec). The most stable isotope is 5H and the least stable isotope is 7H.
Tritium is the most stable radioisotope of hydrogen, with a half-life of 12.32 years. All heavier isotopes are synthetic with half-lives smaller than a zeptosecond (10-21 sec). The most stable isotope is 5H and the least stable isotope is 7H.
![]() Protium, the most prevalent hydrogen isotope, is made up of one proton and one electron. It is the only stable isotope that lacks neutrons.
Protium, the most prevalent hydrogen isotope, is made up of one proton and one electron. It is the only stable isotope that lacks neutrons.
![]() Protium
Protium
![]() 1H is the most abundant hydrogen isotope, accounting for more than 99.98 percent of all hydrogen isotopes. This isotope’s nucleus is made up of a single proton (atomic number = mass number = 1), and its mass is 1.007825 amu.
1H is the most abundant hydrogen isotope, accounting for more than 99.98 percent of all hydrogen isotopes. This isotope’s nucleus is made up of a single proton (atomic number = mass number = 1), and its mass is 1.007825 amu.
![]() As a result, until greater temperatures are attained, H2 dissociates very slightly. The degree of dissociation is only 7.85 percent at 3000K. Because hydrogen atoms are so reactive, they can mix with practically any element.
As a result, until greater temperatures are attained, H2 dissociates very slightly. The degree of dissociation is only 7.85 percent at 3000K. Because hydrogen atoms are so reactive, they can mix with practically any element.
![]() Deuterium
Deuterium
![]() The other stable isotope of hydrogen is 2H, sometimes known as deuterium (D). It has a natural abundance of ~156.25 ppm in the oceans and contributes to about 0.0156 percent of all hydrogen present on the planet.
The other stable isotope of hydrogen is 2H, sometimes known as deuterium (D). It has a natural abundance of ~156.25 ppm in the oceans and contributes to about 0.0156 percent of all hydrogen present on the planet.
![]() The nucleus of deuterium, known as a deuteron, contains one proton and one neutron (mass number = 2), whereas the nucleus of protium, the considerably more common hydrogen isotope, contains no neutrons.
The nucleus of deuterium, known as a deuteron, contains one proton and one neutron (mass number = 2), whereas the nucleus of protium, the considerably more common hydrogen isotope, contains no neutrons.
![]() Deuterium has about twice the mass of protium due to the extra neutron in the nucleus (deuterium has a mass of 2.014102 amu, compared to the mean hydrogen atomic mass of 1.007947 amu).
Deuterium has about twice the mass of protium due to the extra neutron in the nucleus (deuterium has a mass of 2.014102 amu, compared to the mean hydrogen atomic mass of 1.007947 amu).
![]() Deuterium exists naturally in trace amounts as deuterium gas, abbreviated 2H2 or D2, but is most typically found in the universe bound with a protium 1H atom, forming the gas hydrogen deuteride (HD or 1H2H).
Deuterium exists naturally in trace amounts as deuterium gas, abbreviated 2H2 or D2, but is most typically found in the universe bound with a protium 1H atom, forming the gas hydrogen deuteride (HD or 1H2H).
![]() Deuterium reacts chemically similar to ordinary hydrogen (protium), however, there are differences in binding energy and length for compounds of heavy hydrogen isotopes that are bigger than any other element’s isotopic differences.
Deuterium reacts chemically similar to ordinary hydrogen (protium), however, there are differences in binding energy and length for compounds of heavy hydrogen isotopes that are bigger than any other element’s isotopic differences.
![]() Deuterium and tritium bindings are slightly stronger than protium bonds, and these differences are large enough to cause major alterations in biological responses. Deuterium can replace conventional hydrogen in water molecules to generate heavy water (D2O), which is 10.6 percent denser than regular water.
Deuterium and tritium bindings are slightly stronger than protium bonds, and these differences are large enough to cause major alterations in biological responses. Deuterium can replace conventional hydrogen in water molecules to generate heavy water (D2O), which is 10.6 percent denser than regular water.
![]() In eukaryotic animals, heavy water is slightly hazardous, with 25% substitution of the body water causing cell division issues and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure).
In eukaryotic animals, heavy water is slightly hazardous, with 25% substitution of the body water causing cell division issues and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure).
![]() Heavy water consumption does not endanger human health. It is predicted that a 70-kg person can drink 4.8 litres of heavy water without experiencing any adverse effects.
Heavy water consumption does not endanger human health. It is predicted that a 70-kg person can drink 4.8 litres of heavy water without experiencing any adverse effects.
![]() Deuterium is most commonly used in nuclear resonance spectroscopy. Because nuclear magnetic resonance (NMR) needs chemicals of interest to be dissolved in solution, the solution signal should not be analysed.
Deuterium is most commonly used in nuclear resonance spectroscopy. Because nuclear magnetic resonance (NMR) needs chemicals of interest to be dissolved in solution, the solution signal should not be analysed.
![]() Because NMR analyses the nuclear spins of hydrogen atoms, the deuterium nuclear spin property is not seen by the NMR Instrument, making deuterated fluids particularly attractive due to the lack of solvent-signal interference.
Because NMR analyses the nuclear spins of hydrogen atoms, the deuterium nuclear spin property is not seen by the NMR Instrument, making deuterated fluids particularly attractive due to the lack of solvent-signal interference.
![]() Tritium
Tritium
![]() 3H is known as tritium, and its nucleus comprises one proton and two neutrons (mass number = 3). It is radioactive, decomposing into helium-3 by beta decay with an energy release of 18.6 keV. Its half-life is 12.32 years. Tritium is extremely rare on Earth, where it is generated in trace amounts by the interaction of the atmosphere with cosmic rays.
3H is known as tritium, and its nucleus comprises one proton and two neutrons (mass number = 3). It is radioactive, decomposing into helium-3 by beta decay with an energy release of 18.6 keV. Its half-life is 12.32 years. Tritium is extremely rare on Earth, where it is generated in trace amounts by the interaction of the atmosphere with cosmic rays.
Summary
Hydrogen has three naturally occurring isotopes: 1H (protium), 2H (deuterium), and 3H. (tritium). Other highly unstable nuclei (4H–7H) have been generated in the lab but do not occur in nature. Deuterium can replace hydrogen in water molecules to produce heavy water (D2O), which is 10.6% denser than regular water. Heavy water is slightly dangerous in eukaryotic animals, with 25% replacement causing cell division problems and sterility.
Uses for Hydrogen
The most prevalent element in the universe is hydrogen. On Earth, hydrogen is rarely found by itself; it is far more commonly found in chemical combinations with oxygen, carbon, and other elements. Water, for example, is made up of hydrogen and oxygen.
Hydrogen is essential in hydrocarbons like oils, sugars, alcohols, and other organic compounds. Hydrogen is also a “green” energy source; when burned in the air, it produces heat and pure water while emitting no CO2 or other hazardous emissions.
Uses for Deuterium
![]() Although deuterium, often known as “heavy hydrogen,” is naturally occurring, it is less prevalent, accounting for one out of every 6,420 hydrogen atoms. It, like hydrogen, interacts with oxygen to form “heavy water,” which appears and behaves similarly to ordinary water
Although deuterium, often known as “heavy hydrogen,” is naturally occurring, it is less prevalent, accounting for one out of every 6,420 hydrogen atoms. It, like hydrogen, interacts with oxygen to form “heavy water,” which appears and behaves similarly to ordinary water
![]() It is significantly heavier and has a higher freezing point, 3.8 degrees Celsius (38.4 degrees Fahrenheit), compared to 0 degrees Celsius (32 degrees Fahrenheit). Because of the additional neutrons, heavy water is valuable for radiation shielding and other scientific research uses.
It is significantly heavier and has a higher freezing point, 3.8 degrees Celsius (38.4 degrees Fahrenheit), compared to 0 degrees Celsius (32 degrees Fahrenheit). Because of the additional neutrons, heavy water is valuable for radiation shielding and other scientific research uses.
![]() Because heavy water is scarce, it is also significantly more expensive than ordinary water. Its increased weight causes it to behave chemically differently than water. Normal doses are harmless; however, concentrations greater than 25% will harm the blood, nerves, and liver, and extremely high concentrations can be fatal.
Because heavy water is scarce, it is also significantly more expensive than ordinary water. Its increased weight causes it to behave chemically differently than water. Normal doses are harmless; however, concentrations greater than 25% will harm the blood, nerves, and liver, and extremely high concentrations can be fatal.
Uses for Tritium
![]() Tritium is radioactive due to the extra two neutrons it contains, with a half-life of 12.28 years. Tritium cannot be produced naturally, hence it must be produced in nuclear reactors. Although its radiation is potentially harmful, tritium can be beneficial in small doses and with cautious handling and storage.
Tritium is radioactive due to the extra two neutrons it contains, with a half-life of 12.28 years. Tritium cannot be produced naturally, hence it must be produced in nuclear reactors. Although its radiation is potentially harmful, tritium can be beneficial in small doses and with cautious handling and storage.
![]() “Exit” signs built of tritium emit a mellow glow that can last up to 20 years; because they do not require electricity, they provide safety lighting during power outages and other situations. Tritium has various applications in science, such as tracing the flow of water, and it is also used in some nuclear weapons.
“Exit” signs built of tritium emit a mellow glow that can last up to 20 years; because they do not require electricity, they provide safety lighting during power outages and other situations. Tritium has various applications in science, such as tracing the flow of water, and it is also used in some nuclear weapons.
Summary
Hydrogen is rarely found alone on Earth; it is significantly more typically found in chemical combinations with oxygen, carbon, and other elements. Although deuterium, also known as “heavy hydrogen,” occurs naturally, it is rare.
Frequently Asked Questions
Following are some frequently asked questions related to how many neutrons in hydrogen.
1. Which isotopes of hydrogen are radioactive?
It has a half-life of approximately 12.32 years. Tritium is the most stable radioisotope of hydrogen. Tritium, in other words, is the least radioactive of all hydrogen radioactive isotopes. Researchers created four other radioactive hydrogen isotopes, but these isotopes are extremely volatile and simply do not exist.
2. Is Protium an isotope of hydrogen?
Protium is the most prevalent isotope of hydrogen. It accounts for more than 99.98 percent of all hydrogen in the universe. It is referred to as protium since its nucleus contains only one proton. Protium has an atomic mass of 1.00782504(7) u.
3. Are all isotopes are radioactive?
Radioisotopes are elements with atomic numbers larger than 83, which means they have unstable nuclei and are radioactive. They have isotopes (stable nuclei), and most of them have at least one radioisotope (unstable nucleus).
4. Are isotopes dangerous?
Radioactive isotopes are chemical elements generated naturally by the disintegration of atoms. Radiation is often thought to be harmful to the human body, however, radioisotopes are extremely useful in medicine, notably in illness detection and therapy.
5. Why is there no neutrons in hydrogen?
Hydrogen does not have neutrons because its nucleus is so tiny that it cannot house any heavier neutrons. In addition, it makes hydrogen atoms unstable in nature.
6. Can Hydrogen 2 protons?
One proton and one electron make up a hydrogen atom. Hydrogen has an atomic number of one because it has one proton. It is the only element with no neutrons in its atoms. Helium has two protons, two neutrons, and two electrons; it has an atomic number of two.
7. How do u find neutrons?
Subtract the number of protons from the mass number to get the number of neutrons. the amount of neutrons=4019=21
8. Does H+ have a neutron?
In the small nucleus of a hydrogen (H) atom, there are no neutrons. That teeny-tiny atom (the smallest of all) has only one electron and one proton. You can remove an electron and form an ion, but you can’t remove any neutrons. Deuterium is a hydrogen atom that possesses an extra neutron, whereas tritium has two more.
9. What is the mass number of hydrogen?
Hydrogen, the most prevalent element in the universe, has atomic number 1 and an atomic mass of 1.00794 amu, making it the lightest of all known elements. It exists in the form of a diatomic gas (H2).
10. What are protons neutrons?
Protons are subatomic particles that have a positive charge. The strong nuclear force holds protons together in the nucleus of an atom. Neutrons are non-charged subatomic particles (they are neutral).
Conclusion
One proton, one electron, and no neutrons make up a hydrogen atom. A helium atom has two protons and two neutrons in its nucleus, as well as two electrons. When hydrogen is burned in the air, it creates heat and pure water while generating no CO2 or other dangerous emissions.
Tritium is radioactive because it has two extra neutrons, with a half-life of 12.28 years. Although its radiation has the potential to be hazardous, tritium can be useful in tiny doses especially when handled and stored with care.
Nuclear decay photons have higher energy than photons produced by atomic processes, according to physicists. The attractive nuclear contact between nucleons has produced a negative nuclear potential energy–the binding energy EB–that is linked to the missing mass, D m. (the difference between the masses).
Related Articles
How Many Valence Electrons Does Krypton Have
How Many Electrons Does Hydrogen Have
Oxygen atom
Atomic structure