Is O3 a polar or a nonpolar compound? Because of the bent molecular geometry of O3, it is a polar molecule. It’s important to note that because the electric dipole moments of the bonds in O3 are not equal, there is a net dipole moment. As a result, O3 (ozone) is a polar gas in the environment.
Explanation
The bond between the molecule’s three oxygen atoms is what causes it to be polar.
The electron distribution across the molecule is unequal because the centre oxygen atom must exchange electrons with two other bits.
In contrast, the different particles just have to share electrons with one other atom. As a result, the centre atom receives a “lower proportion” of the electron density than the surrounding atoms.
The central atom has a formal charge of +1, whereas the remaining traces each have a standard amount of 12. The -1/2 charge is because the electron density constantly moves across the molecule. Therefore both atoms have the same properties. As a result, the structure of Ozone has a resonance propensity.
O3, often known as Ozone, has a lower melting point of -192°C and a greater boiling temperature of -112°C than comparable molecules such as O2. Because all of the atoms are identical, this is due to a weak polar dipole (oxygen). Ozone is described as a “pale blue” gas with a distinct odour at room temperature and pressure.
Because of the molecule’s high concentration of electronegative (3.44), oxygen atoms are frequently used as an oxidizing agent, swiftly removing electrons from substances/compounds. As a result, Ozone is a volatile molecule that quickly decomposes into O2 at lower atmospheric levels (oxygen).
It is frequently produced by UV radiation and transient electrical bursts in the high atmosphere.
Ozone
Ozone (O3) is a less stable oxygen allotrope than the diatomic molecule (O2). The high atmosphere’s ozone layer prevents potentially deadly UV rays from reaching the Earth’s surface, while ground-level Ozone is an atmospheric pollutant that harms the respiratory system.
Ozone, like water, has a bent structure with C2v symmetry. In the central oxygen, there is only one pair of sp2 hybridization. Because of its bent structure and resonance hybridization, Ozone is a polar molecule (dipole moment = 0.5337 D) .
Ozone is produced when oxygen (O2) is exposed to an electric discharge. Ozone has a unique odour that can be observed following a lightning strike; in fact, the name ozone is derived from the Greek word dozen, which means “to smell.” Ozone can be produced using a graphite rod cathode, a platinum wire anode, and a sulfuric acid (3 M) electrolyte in the lab. The half-cell responses are as follows:
3H2O→O3+6H++6e− (ΔEo=−1.53V)
6H++6e−→3H2 (ΔEo=0V)
6H++6e−→3H2 (ΔEo=0V)
2H2O→O2+4H++4e− (ΔEo=−1.23V)
2H2O→O2+4H++4e− (ΔEo=−1.23V)
Ozone is produced both in the laboratory and in the environment by the photolysis of oxygen.
O2−→hν2O⋅
O2→hν2O⋅
O⋅+O2→O3
O⋅+O2→O3
Ozone is a potent oxidizing agent that may quickly oxidize many materials, including those listed in Equations and It will also corrode metals to their highest oxidation state (except gold, platinum, and iridium), as demonstrated in Equation.
O3+CO→CO2+O2
O3+CO→CO2+O2
O3+2I−+H2O→O2+2OH−+I2
O3+2I−+H2O→O2+2OH−+I2
2Cu+(aq)+2H3O+(aq)+O2(g)→2Cu2+(aq)+3H2O(l)+O2(g)
2Cu(aq)++2H3O(aq)++O2(g)→2Cu(aq)2++3H2O(l)+O2(g)
Metal ozonides, including the ozonide anion (O3-), are explosive and must be stored in a cryogenic environment. There are known ozonides for all of the alkali metals. KO3, RbO3, and CsO3 can be synthesized from their respective superoxides.
KO2+O3→KO3+O2
KO2+O3→KO3+O2
Summary
O3, often known as Ozone, has a lower melting point of -192°C and a greater boiling temperature of -112°C than comparable molecules such as O2. Ozone has a unique odour that can be observed following a lightning strike. It is produced both in the laboratory and in the environment by the photolysis of oxygen.
Molecular Structure Of O3
-
First, let’s take a look at O3’s Lewis structure. The atomic number of oxygen (O) is eight, and it has a valence shell with 6 electrons.
-
The oxygen atom in the centre shares four electrons with the other two oxygen atoms, resulting in one double bond and one single bond.
-
Some people believe that if the centre oxygen atom creates one double bond and one single bond with the other two oxygen atoms, there should be three unbinding electrons left. However, only two unbonded electrons remain on the central oxygen atom.
-
It’s because the O3 has two resonant structures on each side that alternate between double and single bond formation.
-
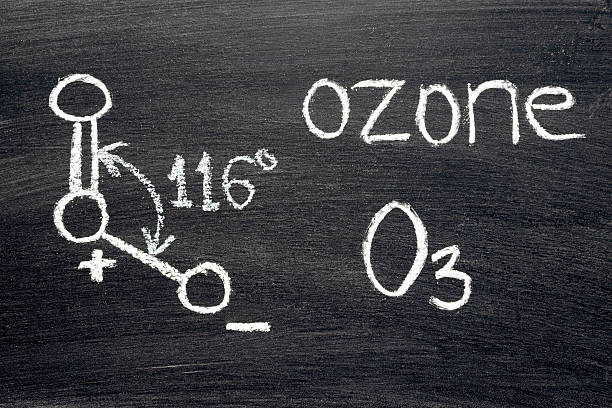
As a result, the Middle Oxygen atom only contains one single pair of electrons. The lone pair is also more repellent to bond pairs on both sides, bending the O3 molecular form. It has a bond angle of 116 degrees created between its bond pairs.
-
The lone pair-bond pair repulsion twists the structure of the O3 molecule, resulting in a net dipole that gives the molecule polarity.
Factors That Alter The Polarity Of A Molecule
Factors that define the polarity of substances are
The Dipole Moment
-
A polar molecule’s net dipole moment is always joyous. Ozone, for example, has a dipole moment of 0.53 D. The Debye, represented by the letter ‘D,’ is the SI unit of dipole moment.
-
The net dipole moment of a nonpolar molecule is equal to 0 D. A molecule’s polarity is proportional to its net dipole moment value.
Electronegativity.
-
The ability to attract electrons is referred to as electronegativity.
-
When the electronegativities of two atoms in a molecule differ, their bond is polar—the more significant the difference in electronegativities between atoms, the greater the total polarity.
-
In the structure of an oxygen molecule, both oxygen atoms have the same electronegativity. As a result, O2 is a nonpolar substance.
Symmetry
-
The polarity and non-polarity of a molecule can also be determined by inspecting its structural geometry. If the molecule has no symmetry, it is a polar molecule; if the shape is symmetrical, it is a nonpolar molecule.
-
The carbon dioxide gas molecule’s C-O bond is polar due to the difference in electronegativity. Due to the symmetry of the CO2 molecule, both C-O bonds are at 180 degrees, cancelling out the polarity. As a result, CO2 has a nonpolar character.
-
We can tell if a molecule is polar or nonpolar by examining these parameters.
Characteristics Of Ozone
-
Sun’s ultraviolet (UV) radiation is blocked by the ozone layer, which shields the Earth from harm. Around the atmosphere spectrum, a layer formed that strongly absorbs UV rays between 220-290 nm.
-
If this layer is not present, UV radiation can harm aquatic life and plants on the Earth’s surface, as well as humans. The ozone layer, on either hand, is being reduced as global warming intensifies.
Summary
The oxygen atom in the centre of the O3 molecule shares four electrons with the other two oxygen atoms, resulting in one double bond and one single bond A molecule’s polarity is proportional to its net dipole moment value. The carbon dioxide gas molecule’s C-O bond is polar due to the difference in electronegativity. If the molecule has no symmetry, it is a polar molecule; if the shape is symmetrical, it’s a nonpolar molecule.
Lewis Structure
Ozone is a well-known example of the Lewis structure. Three oxygen atoms combine to form Ozone. It is designated as O3 in core chemistry equations. Understanding the Lewis structure of the Ozone molecule is crucial for understanding its hybridization, polarity, and molecular geometry.
| Name of molecule | Ozone (O3) |
|---|---|
| No of Valence Electrons in the molecule | 18 |
| Hybridization of O3 | sp2 hybridization |
| Bond Angles | 116 degrees |
| ### Molecular Geometry of O3 | Bent |
Bent Molecular Geometry (O3)
1. Many individuals are now perplexed as to why knowing the Lewis structure of any particular molecule or chemical is required. The explanation is simple: this structure aids in understanding the basic structure and the electrons involved in bond formation and the charges on a specific atom.
2. The octet rule governs lewis’s structure. According to the octet rule, a molecule must contain eight electrons in its outer shell or orbit to be stable. Lewis structure can be used to calculate the number of valence electrons in a molecule. Valence electrons are electrons that participate in bond formation and nonbonding electron pairs.
3. Bonding pairs of electrons are electrons that contribute to the formation of a bond. Lines in the Lewis structure represent these electrons. Nonbonding electron pairs are electrons that do not participate in the shape of bonds. The electrons are represented by the dots that surround the central atom.
O3 Valence Electrons
-
Each Oxygen molecule in Ozone or O3 has six valence electrons.
-
Because there is three oxygen molecules, the total number of valence electrons is 6*3=18.
-
As a result, the Ozone molecule possesses a total of 18 accessible valence electrons.
O3 Lewis Structure
Because this structure follows the octet rule, the central atom should first have eight electrons in its outer shell. As a result, one oxygen molecule is in the centre while the other two are on opposing sides.
The centre atom possesses one lone pair of electrons due to the eight electrons in its outermost orbit and is stable. To satisfy the octet rule, a central bit must form a double bond on one of its sides with an Oxygen molecule and another single bond on another. Because both Oxygen molecules have the same electronegativity and structure, the double bond between them continues to move.
Resonant Structures Of O3
Ozone’s core atom, on the other hand, has one double bond and one single bond with its neighbouring oxygen molecules, giving it a one-of-a-kind structure. The Ozone has a resonant Lewis structure because these bonds are constantly swapping locations.
Resonance refers to the continuous interchanging of bonds between the three Ozone molecules. The core atom of the Lewis structure will have a charge of +1, whereas the bit making a single bond will have an account of -1.
O3 Hybridization
One definition of hybridization is the creation of a new chemical orbital by mixing together atomic levels with similar or dissimilar energies. Once we know the Lewis structure of a molecule, we may quickly determine its hybridization.
Because Ozone contains only one central Oxygen atom with eight electrons in its outermost shell, the hybridization of the central atom will be sp2. The 2s orbital has two electrons, while each of the three 2p orbitals has six electrons. Because there are electrons in one s orbital and two p orbitals, the hybridization of the centre oxygen atom is sp2.
The other two oxygen atoms have similarly undergone hybridization. Because only one lone pair of electrons generates resonance in the Ozone structure, one will have sp2 hybridization, and the other will have sp3 hybridization. Ozone has sp2 hybridization because the hybridization of the centre atom is usually considered the ultimate hybridization.
O3 Molecular Geometry
Because the hybridization of the molecule governs its shape, we can now establish the molecular geometry of Ozone. Ozone should have a trigonal planar shape because it is sp2 hybridized.
Because Ozone possesses resonance and one lone pair of electrons, the angle between the molecules is less than 120 degrees.
O3 Bond Angles
There is always a repulsive force between bonding electrons that is less than the repulsive force between a lone pair and bonding electrons. Because there is just one pair of lone electrons in this circumstance, the angle decreases from 120 to 116 degrees.
O3 Form
This decrease in angle from 120 to 116 degrees causes a bend in shape, resulting in a distorted trigonal planar shape. Because of its distortion, the Ozone formation is frequently regarded as curved or planar.
A molecule’s polarity is determined by its molecular geometry. Because of its valence electrons, the Ozone molecule is twisted here. Because of their sp2 hybridization, all three Oxygen molecules are not linear.
Because the molecules are not in linear geometry, their dipole interactions are not neutralized, and as a result, this molecule has a net dipole. Thus, there is polarity in Ozone, and it is possible to assert that Ozone is polar. This polarity is caused by a single pair of electrons on the core of the Ozone atom.
Summary
Ozone is made up of 18 valence electrons, one of which is a lone pair of electrons, and it has a valence of 18. Among its characteristics include polarity, sp2 hybridization, and trigonal-planar geometry (bent/angular).
Ozone As A Modulator Of Earth’s Life
The Earth’s atmosphere serves as a source of O2 and a sink for CO2, but it also acts as a barrier to life. To begin with, practically all meteorites burn up upon arrival because of the extremely high temperatures created by atmospheric friction. Second, the atmosphere acts as a barrier against high-energy UV rays.
Although UV light converts 7-dehydrocholesterol into vitamin D3 in the skin and is thus beneficial, high-intensity UV harm live cells. In truth, the darkening we call a suntan is the body’s way of protecting itself from more UV harm.
Sunburn and skin cancer are caused by relatively mild UV light that reaches the Earth’s surface; without the atmosphere, humans would be subjected to high-intensity UV radiation, which would endanger all life on Earth. Ozone (O3) is the Earth’s “sunscreen” (O3). And there would be no life on Earth if there was no ozone in the upper atmosphere.
In the lower stratosphere, from 10 and 50 kilometers above the Earth, the ozone layer is present, but its thickness varies seasonally and geographically.
This layer contains approximately 91 percent of the Ozone in the Earth’s atmosphere and absorbs between 93 and 99 percent of the sun’s high-frequency UV light. Ozone decomposes photolytically to O2 and molecular oxygen (9.2.10), and it is this reaction that accounts for the atmosphere’s UV protection. The exothermic reaction of molecular oxygen with O2 naturally regenerates Ozone.
O3+hν→O2+O
O3+hν→O2+O
O+O2→O3
O+O2→O3
The balance between ozone formation and destruction is critical for protecting the planet’s living species. While the Earth’s ozone layer has been relatively stable for millions of years, the last 70 years have seen significant changes, including widening the polar hole in the ozone layer.
Geographically, the ozone hole is defined as an area with a total ozone concentration of less than 220 Dobson Units.
One Dobson unit is equivalent to a layer of Ozone 10 m (micrometre) thick at standard temperature (25 °C) and pressure (1 atmosphere) (1 atmosphere).
Over the last two and a half decades, the ozone hole has expanded in size and duration. The current size of the ozone hole above Antarctica is estimated to be around 30 million square kilometres.
Frequently Asked Questions
People have asked these questions frequently
1. What makes O3 a polar molecule?
The bond between the molecule’s three oxygen atoms is what causes it to be polar. The electron distribution across the molecule is unequal because the centre oxygen atom must exchange electrons with two other bits. In contrast, the different particles just have to share electrons with one other atom.
2. Is O3 polar and linear?
Yes, O3 had three solitary pairs in the equatorial location. However, because they are similarly spaced, they repel the axial I, in the same way, resulting in a linear shape.
3. Does O2 have polar or nonpolar properties?
For example, molecular oxygen (O2) is nonpolar because the electrons are distributed evenly between the two oxygen atoms. Methane (CH4) is another example of a nonpolar covalent bond (CH4)
4. Is O3 an ionic or a covalent compound?
Covalent bonding exists in Ozone. This is because the sharing of electrons inside them links atoms in Ozone.
5. What is the definition of O3 molecular geometry?
O3 has a trigonal planner molecular geometry.
The electrons in the two bonds are repelled by the existence of a lone pair on the core metal atom, which causes the bit to adopt a bent molecular geometry.
6. What is O3’s electronegativity?
The two OO bonds in ozone (O3) molecules are nonpolar (there is no electronegativity difference between atoms of the same element) (there is no electronegativity difference between atoms of the same part).
7. Is there a net molecular dipole in O3?
The computation of formal charges reveals that the centre O has a +1 control, but the O atom with the single bond has a -1 account. Because Ozone has a bent form, it possesses a net dipole moment.
8. What is the intermolecular force of O3?
The intermolecular interactions in the ozone molecule are not particularly strong. The London-dispersion force is the operational force. The molecule also has a coordinate bond. Because of its weak intermolecular interactions, it has a low melting and boiling point.
9. What kind of bond is 03?
Has a double bond and two lone electron pairs. It gives the third O atom one of the lone pair. As a result, O3 is formed by a double bond, which is a coordinate covalent link.
10. Is there a net molecular dipole in O3?
The computation of formal charges reveals that the centre O has a +1 control, but the O atom with the single bond has a -1 control. Because Ozone has a bent structure, it possesses a net dipole moment.
Conclusion
Ozone (O3) is a polar compound. The three oxygen atoms’ connection gives the molecule polarity. An odorous “light blue” gas UV light creates ozone in the lab and outdoors. Ozone Research Center (ODRC). OXIDATES METAL (except gold, platinum, and iridium). So O3 bends. CO2’s C-O bond is polar due to atom electronegativity.
CO2 is nonpolar due to symmetry. Hybridization, polarity, and molecular geometry knowledge are necessary. Each electron in the outer shell or orbit must be stable. Valves produce bonds and no bonds. Oddly, ozone’s structure allows for bond swapping. The shape of a molecule is determined by hybridization.
Ozone only has one electron pair. Ozone has one lone electron. I, like Ozone, am polar. It blocks 93-99% of the sun’s radiation. Antarctica ozone hole 30 million sq. km at 25°C, a 10 m (micrometre) thick Ozone layer (1 atmosphere). In the bit’s chemical structure, a single metal atom repels electrons from two bonds.