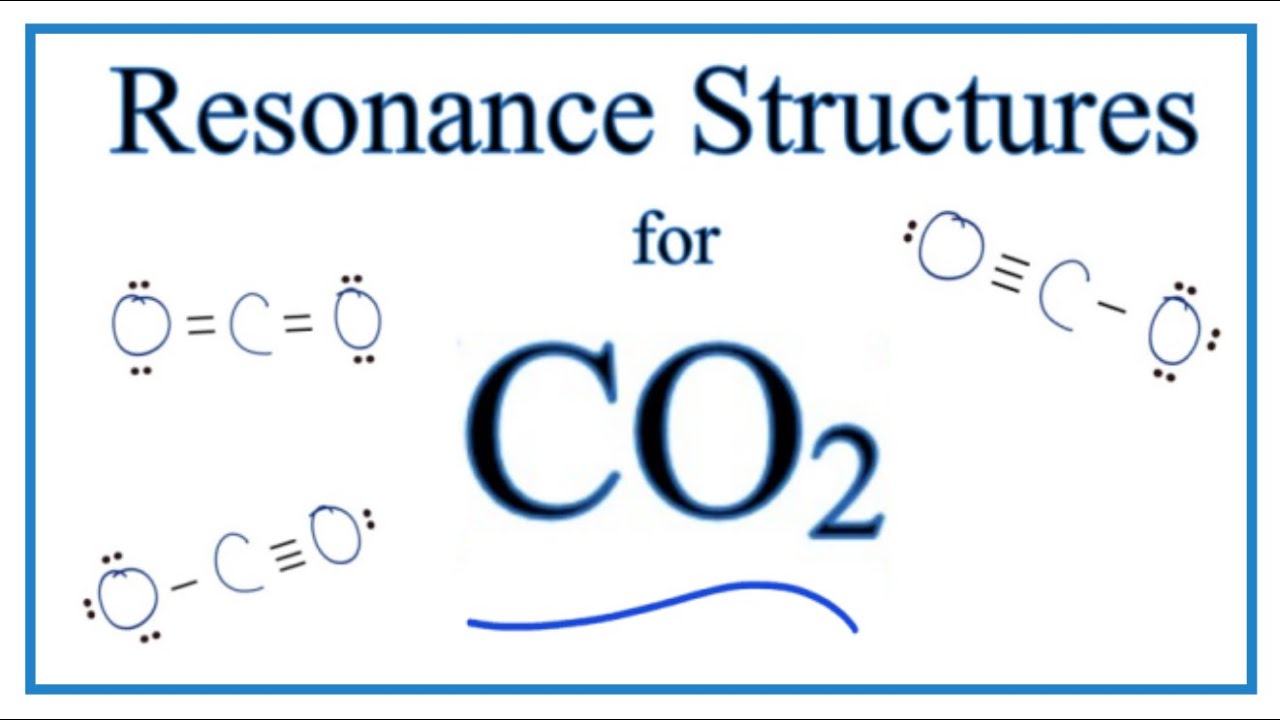
The CO2 resonance structure are 3 in number, one of which is a substantial contribution. The carbon dioxide molecule has 16 valence electrons, four from carbon and six from each oxygen atom. The lewis structure for CO2 has two double bonds between carbon and oxygen. This completes the carbon’s valence of four. Each oxygen atom is surrounded by two lone pairs.
CO2 Lewis Structure And Shape:
There are two double bonds around the carbon atom in the Lewis structure of CO2. Each oxygen atom possesses two lone pairs, while the carbon atom has none. Furthermore, oxygen and carbon atoms have no charges.
When it comes to the shape, there are two sigma bonds surrounding the carbon atom, and it does not have any lone pairs. As a result, the form should be linear around the carbon atom.
The Total Number Of Electrons In The Valance Shell Of CO2:
Carbon dioxide is made up of the elements oxygen and carbon. Oxygen is a member of the VIA group and has six electrons in its final shell. Carbon has four electrons in its valence shell and belongs to group IVA. The number of electrons in the valence shells of the oxygen atom is currently known.
To calculate the total number of valence electrons given by an element, multiply the number of electrons in the valance shell by the number of atoms in that element.
• oxygen provide 6 * 2 = 12 valence electrons
• The number of valence electrons provided by the carbon atom is 4 * 1 = 4.
•12 + 4 = 16 total valence electrons
Carbon Dioxide:
Carbon dioxide (CO2) is a chemical molecule that exists as an acidic colorless gas with a density that is roughly 53% higher than that of dry air. In a carbon dioxide molecule, a carbon atom is covalently connected to two oxygen atoms. It is found as a trace gas in the Earth’s atmosphere.
By volume, the current concentration is 0.04 percent (412 parts per million), up from 280 parts per million before the industrial revolution. Volcanoes, forest fires, hot springs, and geysers are all natural sources, and it is freed from carbonate rocks by dissolving in water and acids.
Carbon dioxide can be found in groundwater, rivers and lakes, ice caps, glaciers, and the ocean since it is soluble in water. Petroleum and natural gas reserves contain it. Carbon dioxide has a harsh, acidic odour and in the mouth tastes like soda water. At normal quantities, however, it has no odour.
Carbon dioxide is the principal source of accessible carbon in the carbon cycle, and its concentration in the pre-industrial atmosphere has been regulated since late in the Precambrian by photosynthetic organisms and geological events.
Photosynthesis is a process in which plants, algae, and cyanobacteria use sunlight to synthesise carbohydrates from carbon dioxide and water, with oxygen as a byproduct.
When aerobic organisms break down organic molecules for energy, oxygen is consumed and CO2 is produced as a waste product. CO2 is necessary for life on Earth since plants require it for photosynthesis and humans and animals rely on plants for nourishment.
Fish gills return it to the water, and air-breathing terrestrial species, including humans, return it to the air. The breakdown of organic components and the fermentation of carbohydrates produce carbon dioxide in the production of bread, beer, and wine.
It is made from wood, peat, and other organic resources, as well as fossil fuels such as coal, petroleum, and natural gas. It’s an unwanted byproduct of several large-scale oxidation processes, such as the manufacture of acrylic acid (which produces over 5 million tonnes per year).
It’s a versatile industrial material used in a number of applications, including welding and fire extinguishers, pressurising gas in air guns and oil recovery, chemical feedstock, and supercritical fluid solvent in coffee decaffeination and supercritical drying.
It is used to make drinking water and carbonated beverages like beer and sparkling wine effervescent. Dry ice is a frozen solid form of CO2 that is used in dry-ice blasting as a refrigerant and abrasive.
Summary:
There are two double bonds around the carbon atom in the Lewis structure of CO2. Carbon dioxide has a harsh, acidic odour and in the mouth tastes like soda water.In a carbon dioxide molecule, a carbon atom is covalently connected to two oxygen atoms.Carbon dioxide (CO2) is the most major long-lived greenhouse gas.
Physical Properties
Carbon dioxide has no color. At low concentrations, the gas is odourless, but at sufficiently high concentrations, it emits a pungent, acidic stench. [At standard temperature and pressure, carbon dioxide has a density of around 1.98 kg/m3, which is about 1.53 times that of air.
At pressures below 0.51795(10) mpa[2] (5.11177(99) atm), carbon dioxide has no liquid state. The gas becomes a solid at temperatures below 194.6855(30) K (78.4645(30) °C at a pressure of 1 atm (0.101325 mph), and the solid becomes a gas at temperatures beyond this. When carbon dioxide is solid, it is referred to as dry ice.
Only at pressures greater than 0.51795(10) mpa[2] (5.11177(99) atm does liquid carbon dioxide form; the triple point of carbon dioxide is 216.592(3) K (56.558(3) °C) at 0.51795(10) mpa[2] (5.11177(99)atm).
At 7.3773(30) mpa[2] (72.808(30) atm, the critical point is 304.128(15) K[2] (30.978(15) °C). An amorphous glass-like solid of solid carbon dioxide has also been recorded at high pressure. Carbonia is made by supercooling heated CO2 in a diamond anvil at extremely high pressures (40–48 GPA, or about 400,000 atmospheres).
This discovery supported the notion that carbon dioxide, like other members of its elemental family such as silicon (silica glass) and germanium dioxide, could exist in a glass state.
Applications:
The food sector, the oil industry, and the chemical industry all use carbon dioxide. The chemical has a variety of commercial uses, but one of the most important is in the production of carbonated beverages; it is responsible for the sparkle in soda water, beer, and sparkling wine.
Chemicals’ Precursor
In the chemical industry, carbon dioxide is mostly used to make urea, with a tiny quantity also being used to make methanol and a variety of other chemicals. The Kolbe-Schmitt technique is used to make a variety of carboxylic acid derivatives, including sodium salicylate.
Electrochemical techniques are being investigated at the research level in addition to traditional CO2 processes for chemical synthesis.
Agriculture
Carbon dioxide is required for photosynthesis in plants. Because very high carbon dioxide concentrations (100 times atmospheric concentration or more) can be hazardous to animal life, boosting the concentration to 10,000 ppm (1 percent) or higher for many hours will kill pests such as whiteflies and spider mites in a greenhouse.
Foods
Carbon dioxide is a food ingredient that is used as a propellant and acidity control in the food business. It has been approved for use in the EU (E number E290), the United States, Australia, and New Zealand (listed by its INS number 290).
Pop Rocks are a candy that is compressed with carbon dioxide gas at 4,000 kph (40 bar; 580 psi). When you put it in your mouth, it melts and explodes, releasing the gas bubbles (just like other hard candy).
Because leavening agents produce carbon dioxide, the dough rises.Chemical leaveners like baking powder and baking soda release carbon dioxide when heated or exposed to acids, whereas baker’s yeast produces carbon dioxide by digesting carbohydrates in the dough.
Beverages
Carbon dioxide is utilised in the production of carbonated beverages and soda water. Beer and sparkling wine have traditionally been carbonated through natural fermentation, but many manufacturers now use carbon dioxide collected from the fermentation process to carbonate these beverages.
Recycled carbon dioxide is the most frequent method of carbonation in bottled and kegged beer. Draught beer is often carried from kegs in a cold room or basement to dispensing taps on the bar using pressurised carbon dioxide, which is occasionally coupled with nitrogen.
The flavour of soda water (and similar taste sensations in other carbonated beverages) comes from dissolved carbon dioxide rather than bursting bubbles in the gas.
Carbonic anhydrase 4 converts carbonic acid to carbonic acid, which gives the dissolved carbon dioxide a sour flavour and a tactile sensation.
During the cold soak phase of winemaking, carbon dioxide in the form of dry ice is commonly used to immediately freeze clusters of grapes after picking to assist prevent spontaneous fermentation by wild yeast.
The key benefit of utilising dry ice instead of water ice is that it cools the grapes without adding any additional water, which could diminish the sugar content of the grape must and, as a result, the alcohol content of the finished wine.
Carbon dioxide is also utilised to produce a hypoxic atmosphere for the carbonic maceration process, which is how Beaujolais wine is made.
Carbon dioxide is occasionally used to prevent wine bottles or other storage vessels, such as barrels, from oxidising, but it has the drawback of dissolving in the wine and making a previously still wine slightly frothy. As a result, professional winemakers employ nitrogen or argon as an alternative gas in this process.
Stunning Animals
Before slaughter, carbon dioxide is frequently used to “stun” animals. The term “stunning” may be misleading, as the animals are not immediately knocked out and may experience distress.
Inert Gas
Carbon dioxide is one of the most commonly used compressed gases in pneumatic (pressurised gas) systems in portable pressure tools.Carbon dioxide is employed as a welding environment, despite the fact that it oxidises most metals in the welding arc.
Despite evidence that carbon dioxide welds are more brittle than welds performed in more inert environments, carbon dioxide is commonly employed in the automotive sector. When used for MIG welding, CO2 is referred known as MAG welding, or Metal Active Gas, since it may react at these high temperatures.
It creates a hotter puddle than inert atmospheres, which improves flow quality. This could, however, be owing to air processes near the puddle.
This has the opposite impact when welding because it tends to embrittle the site, but it may not be an issue for conventional mild steel welding when final ductility isn’t a key concern.
For fast inflation, life jackets frequently include pressurised carbon dioxide canisters. CO2 capsules can also be used to manufacture carbonated water and to fill air pistols, paintball markers/guns, and bicycle tyres.
Carbon dioxide can also be used to kill pests in large numbers.Supercritical drying of some culinary and technical materials, specimen preparation for scanning electron microscopy[60], and decaffeination of coffee beans all employ liquid carbon dioxide.
Summary:
Carbon dioxide has a density of 1.98 kg/m3 at standard temperature and pressure, which is about 1.53 times that of air.At low concentrations, the gas is odourless, but at sufficiently high concentrations, it emits a pungent, acidic stench. The presence of carbon dioxide is required for photosynthesis in plants.At the research level, electrochemical techniques are being examined.
Fire Extinguisher
Flames can be extinguished by flooding the area around the flame with carbon dioxide. Rather than extinguishing the flame, moving it deprives it of oxygen.
Carbon dioxide extinguishers are effective in extinguishing small flammable liquid and electrical fires, but they are ineffective in extinguishing larger combustible fires because they do not significantly cool the burning substances and can catch fire once the carbon dioxide has evaporated.
For localised threats and total flooding of a protected zone, carbon dioxide has also been used as an extinguishing chemical in stationary fire-fighting systems.
Carbon-dioxide systems for fire protection of ship holds and engine rooms are recognised by International Maritime Organization standards. Carbon-dioxide-based firefighting systems have been related to multiple deaths, as high quantities of the gas can induce suffocation.
Between 1975 and the study’s date (2000), there were 51 CO2 system incidents, resulting in 72 deaths and 145 injuries.
Biological Role:
Carbon dioxide is produced as a consequence of cellular respiration, which happens when organisms break down carbohydrates, lipids, and amino acids for energy using oxygen. All plants, algae, and animals, as well as aerobic decay and bacteria, are included.
Carbon dioxide passes through the blood from the body’s tissues to the skin (e.g., amphibians) or gills (e.g., fish), where it dissolves in the water, or to the lungs, where it is expelled invertebrates. Plants can take more carbon dioxide from the atmosphere during active photosynthesis than they release during respiration.
Photosynthesis And Carbon Fixation
Plants, algae, and (cyanobacteria) transform atmospheric carbon dioxide into energy-rich organic compounds like glucose, allowing them to create their food through photosynthesis.
From carbon dioxide and water, photosynthesis produces sugars that can be utilised to make a variety of organic compounds, as well as oxygen as a byproduct.
As indicated in the diagram at left, ribulose-1,5-bisphosphate carboxylase oxygenase (rubisco) is the enzyme engaged in the first major stage of carbon fixation, the creation of two molecules of 3-phosphoglycerate from CO2 and ribulose bisphosphate.
Rubisco is estimated to be the most common protein on the planet. Photosynthetic products are used by phototrophs as both internal food and raw materials for the production of more complex organic compounds such as polysaccharides, nucleic acids, and proteins.
These are used for their growth as well as to form the foundation of food chains and webs that feed other organisms, including humans. Hard calcium carbonate scales are produced by coccolithophores, which are important phototrophs.
Emiliania huxleyi is a well-known coccolithophore whose calcite scales have served as the foundation for a variety of sedimentary rocks, including limestone, where previously atmospheric carbon can be retained across geological timescales.
Plants can grow up to 50% quicker under 1,000 ppm CO2 concentrations than in ambient circumstances, albeit this implies no change in climate and no limitation on other nutrients. Greater CO2 levels produce increased growth, which is reflected in crop harvestable yield, with wheat, rice, and soybean all showing yield increases of 12–14 percent in FACE trials.
Increased CO2 concentrations in the atmosphere cause fewer stomata to grow on plants, resulting in lower water consumption and higher water efficiency.CO2 enrichment results in lower micronutrient concentrations in crop plants, according to FACE studies.
Herbivores will need to eat more food to get the same quantity of protein, which may have knock-on consequences on other sections of ecosystems. Secondary metabolite concentrations, such as phenylpropanoids and flavonoids, can also be altered in plants exposed to high CO2 levels.
Because C3 photosynthesis plants and algae also exhale CO2 during respiration, the bulk of C3 photosynthesis plants and algae are only net absorbers during the day.
Despite the popular belief that mature forests are carbon-neutral, they can continue to accumulate carbon and serve as essential carbon sinks, assisting in the maintenance of the Earth’s carbon balance. Furthermore, phytoplankton photosynthesis consumes dissolved CO2 in the upper water, encouraging CO2 absorption from the atmosphere, which is important for life on Earth.
Toxicity
Carbon dioxide content in fresh air ranges between 0.036 percent (360 ppm) and 0.041 percent (412 ppm) depending on location (averaged between sea level and 10 kph level, i.e., around 30 km (19 mi) height).
CO2 is an asphyxiant gas that is not classed as dangerous or harmful by the United Nations Economic Commission for Europe’s Globally Harmonized System of Classification and Labelling of Chemicals standards, which are based on the OECD Guidelines for Chemical Testing.
It can make some people tired and give their lungs a congested feeling at concentrations up to 1% (10,000 ppm). Even in the presence of sufficient oxygen, concentrations of 7% to 10% (70,000 to 100,000 ppm) can produce asphyxia, which manifests as dizziness, headache, vision and hearing impairment, and coma within minutes to hours.
Hypercapnia, a subset of asphyxiation, is the word used to describe the physiological effects of acute carbon dioxide exposure.
Because it is heavier than air, it can collect in sheltered/pocketed locations below average ground level in locations where the gas seeps from the ground in relatively high concentrations (due to sub-surface volcanic or geothermal activity) without the dispersing effects of wind, causing animals to suffocate.
Carrion eaters drawn to the carcasses are subsequently killed as well. CO2 emissions from the adjacent volcano Mount Nyiragongo have killed children in the same way near the city of Goma. ‘Mazuku’ is the Swahili word for this phenomenon.
Humans adapt to higher CO2 concentrations by changing their breathing patterns and producing renal bicarbonate to counteract the effects of blood acidification (acidosis).
Several studies have suggested that 2.0 percent inspired concentrations could be employed in tight air spaces (such as a submarine) because the adaptation is physiological and reversible, and there is no impairment in performance or regular physical activity after five days of exposure.
Other studies, on the other hand, demonstrate a decline in cognitive performance at far lower levels. Furthermore, adaption or compensating mechanisms will be unable to reverse the disease if respiratory acidosis persists.
Resonance:
The phenomenon of increased amplitude is known as resonance when the frequency of a periodically applied force (or a Fourier component of it) is equal to or close to the natural frequency of the system it affects.
When a resonant frequency oscillating force is applied to a dynamic system, the system oscillates with greater amplitude than when the same force is applied at non-resonant frequencies. The system’s resonant frequencies, also known as resonance frequencies, are the frequencies at which the response amplitude is at its maximum.
Small periodic stresses at the system’s resonance frequency can cause considerable amplitude oscillations due to the storage of vibrational energy.
Mechanical resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance (ESR), and quantum wave function resonance are all examples of resonance occurrences.
Resonant systems can be used to generate vibrations of a certain frequency (for example, musical instruments) or to choose specific frequencies from a complex vibration with various frequencies (for example, computers) (e.g., filters).
The term resonance (from Latin resonant, ‘echo,’ from resonate,’ resound’) comes from the science of acoustics, specifically sympathetic resonance, which may be seen in musical instruments when one string begins to vibrate and generate a sound when another is struck.
Types:
Mechanical And Acoustic
A mechanical system’s tendency to absorb more energy when the frequency of its oscillations matches the system’s inherent frequency of vibration is known as mechanical resonance. In inadequately designed structures such as bridges, buildings, trains, and aircraft, it can induce extreme swaying motions and even catastrophic failure.
Engineers must ensure that the mechanical resonance frequencies of parts do not coincide with the driving vibrational frequencies of motors or other oscillating elements when constructing items to avoid a phenomenon known as resonance disaster.
Every skyscraper, tower, and bridge construction project must consider how to avoid resonance tragedies. Shock mounts can be qualified as a countermeasure to absorb resonant frequencies and disperse the absorbed energy.
To cancel resonance, the Taipei 101 building has a 660-tonne pendulum (730-short-ton)—a tuned mass damper. In addition, the construction is engineered to resonate at a frequency that isn’t common. In seismic zones, buildings are frequently built to accommodate the oscillating frequencies of expected ground motion.
Engineers must also verify that the mechanical resonance frequencies of parts do not match the driving vibrational frequencies of motors or other strongly oscillating parts when constructing items with engines.
Mechanical resonance in a balance wheel, pendulum, or quartz crystal keeps time in clocks. Due to the resonance between the elastic energy stored in the lower limb and the mass of the runner, the cadence of runners has been postulated to be energetically beneficial.
Acoustic resonance is a subset of mechanical resonance that deals with mechanical vibrations in the range of human hearing, or in other words, sound. Hearing is generally restricted to frequencies between 20 Hz and 20,000 Hz (20 kHz) in humans.
Many items and materials with resonance frequencies in this range act as resonators, vibrating mechanically and pushing on the surrounding air to create sound waves when struck. Many of the percussive sounds we perceive come from this source.
Because most acoustic instruments use resonators, such as the strings and body of a violin, the length of tube in a flute, and the form of, and stress on, a drum membrane, acoustic resonance is an important issue for instrument builders.
Acoustic resonance, like mechanical resonance, can cause the catastrophic breakdown of the object at resonance. Breaking a wine glass with sound at the exact resonance frequency of the glass is a typical example, however, this is impossible in practice.
International Space Station
An autopilot controls the rocket engines for the International Space Station (ISS). Normally, uploaded parameters for regulating the Zvezda module’s engine control system cause the rocket engines to propel the International Space Station into a higher orbit.
The rocket engines are hinge-mounted, and the crew is usually unaware of the action. The uploaded parameters, on the other hand, caused the autopilot to swing the rocket engines in increasing and larger oscillations at a frequency of 0.5 Hz on January 14, 2009. These oscillations lasted 142 seconds and were captured on camera.
Electrical
Electrical resonance occurs at a given resonant frequency when the impedance of an electric circuit is at a minimum in a series circuit or at a maximum in a parallel circuit (usually when the transfer function peaks in absolute value).Resonance in circuits is utilized in wireless communications such as television, cell phones, and radio to broadcast and receive data.
Summary:
Carbon dioxide extinguishers are effective on small flammable liquid and electrical fires, but ineffective on larger combustible fires. They do not considerably cool the burning material, and once the carbon dioxide has dissipated, when exposed to ambient oxygen, they can catch fire. Rather than extinguishing the flame, moving it deprives it of oxygen.
Frequently Asked Questions:
Following are the questions related to this keyword
1: Does CO2 have resonance?
Carbon dioxide (CO2) has three resonance structures, one of which is very important. There are 16 valence electrons in the CO2 molecule, four from carbon and six from each oxygen atom.
2: What is the structure of CO2?
The carbon and oxygen atoms in the carbon dioxide molecule form two double bonds. One sigma and one pi link make up each double bond, therefore a carbon dioxide molecule has two sigma and two pi bonds.
3: What is the electron dot structure for CO2?
The valence electrons in an oxygen atom form two lone pairs. It must create a double bond because it is only linked to one carbon atom. There are no lone pairs in the carbon atom because it has four valence electrons. As a result, each oxygen atom is twice bound to it.
4: How many bonding electrons does CO2 have?
When the four electrons from the valence shells are combined to the four electrons from the oxygen atoms, the CO2 molecule possesses a total of eight electrons. Because carbon has no charge, no additional electrons are required, resulting in an overall total of 8.
5: How many molecular orbitals does CO2 have?
CO2 has two sigma molecular orbitals with ag and b1u symmetry, as well as two molecular orbitals with b2u and b3u symmetry. Each of these bonding molecular orbitals has an antibonding partner with higher energy.
6: What is a CO2 molecule?
The molecule carbon dioxide has the chemical formula CO2.Carbon dioxide, or CO2, is a colourless gas. It’s made up of two oxygen atoms that are covalently bound to a single carbon atom. Animals exhale it, whereas plants use it during photosynthesis. Carbon dioxide is a chemical that is colorless and odorless.
7: Is CO2 an ionic or covalent bond?
CO2 isn’t an ionic substance at all. An ionic compound is usually generated between a metal atom and a non-metal atom, according to the definition. CO2, on the other hand, is a covalent chemical that is created between two non-metal atoms (carbon and oxygen).
8: How does CO2 affect the atmosphere?
It absorbs less heat per molecule than the greenhouse gases methane and nitrous oxide, but it’s more abundant and stays in the atmosphere for much longer. Carbon dioxide emissions account for over two-thirds of the total energy imbalance that is causing global warming.
9: Does CO2 have a triple bond?
The CO2 molecule’s initial VSEPR shape is Tetrahedral. For each multiple bond (double/triple bond), subtract one electron from the final amount. The CO2 molecule subtracts two electrons from the total since it has two double bonds.
10: What happens to the CO2 that is produced during cellular respiration?
Carbon dioxide is emitted as a waste product during the process of cellular respiration. Photosynthesizing cells can utilize this carbon dioxide to make new carbohydrates.
Conclusion:
Carbon dioxide (CO2) has three resonance structures, one of which is very important. There are 16 valence electrons in the CO2 molecule, four from carbon and six from each oxygen atom. CO2 has two bonding molecular orbitals with an antibonding partner with higher energy. The CO2 molecule is made up of two oxygen atoms that are covalently bound to a single carbon atom.