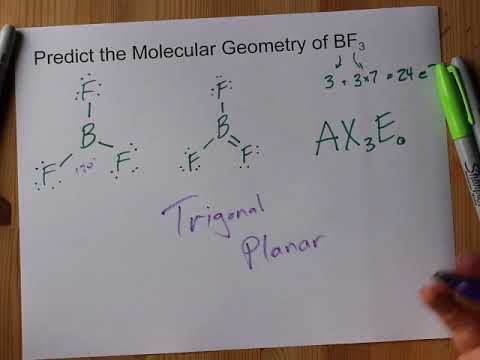
BF3 molecular shape is called trigonal planar. At the corners of an equilateral triangle, fluorine atoms are situated. Each of the four atoms lies in the same plane because the F-B-F angle is exactly 120 degrees, making BF3 molecular shape.
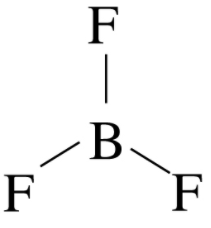
Molecular Geometry of BF3
BF3’s molecule has a “Trigonal Planar” geometry. A “Trigonal Planar” model in chemistry has three atoms arranged in a circle around a central atom. Because all three of them have 120° bond angles, they form an equilateral triangle, making it look like they’re all in the same plane.
What is BF₃ and how is it formed?
One of the most common names for the gaseous boron trifluoride, BF3, is Boron Trifluoride. Many other Boron compounds can be made from this inorganic compound, but it is extremely toxic. That’s when it comes into being. When three’sp2’ hybrid orbitals of Boron bond with three ‘p2’ hybrid orbitals of Fluorine, BF3 is formed. This can be explained in terms of its hybridization.
BF3 Lewis Structure
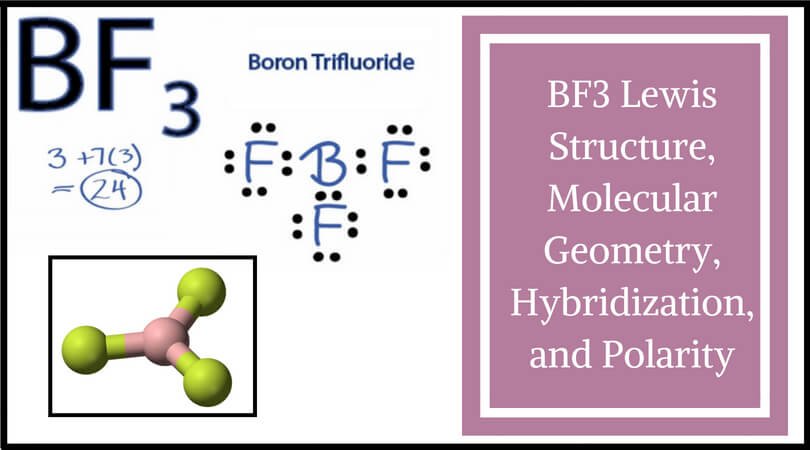
Calculating the number of valence electrons in the BF3 molecule is the first step in determining its Lewis structure. We must arrange the valence electrons in a circle around the central atom of BF3 because it has a total of 24 of them. Don’t forget to figure out how many valence electrons Boron Trifluoride has before completing the octets.
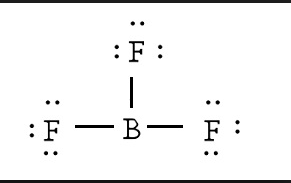
To put it another way, boron is the least electronegative element. Six valence electrons must be present in the outer shell. Even though boron only had six valence electrons, we can verify that the formal charges in the Boron Trifluoride Lewis structure are zero.
How to Draw BF3 Lewis Structure?
There are several steps involved in drawing a Lewis structure. A total of 24 electrons can be found here. In order to finish it, you’ll need to give the outer atom more octets and the central atom more electrons. In reality, there are no more electrons than what we already have. The result of subtracting 24 from 24 is zero.
Violations
When drawing Lewis structures, it’s important to remember that the Octet Rule can be broken in any of these three ways. However, since this is an uncommon occurrence, it’s not necessary to keep this in mind all the time.
BF3 Hybridization
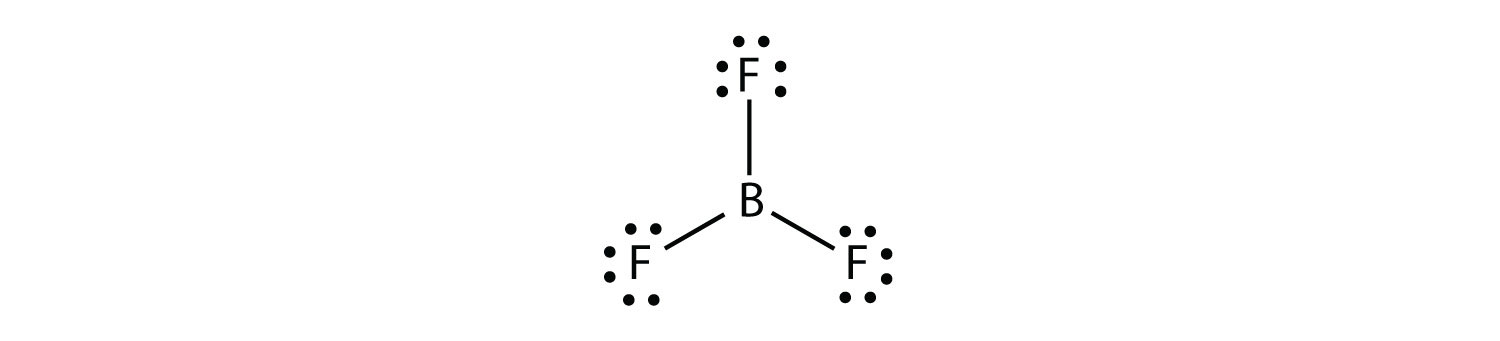
When atomic orbitals are combined into new hybrid orbitals, the term “hybridization” is used. Molecular geometry and nuclear bonding properties can be explained in a way that is amenable to these theories. BF3 is an SP2 hybridization, one of several types of hybridization.
There are only three atoms of boron in the SP2 molecule, so one (pi) bond is needed to form the double bond between them. There are three equivalent SP2 hybrid orbitals formed by the atomic S and P orbitals in Boron’s outer shell.
BF3 Polarity
Polarity refers to a molecule or its groups having an electric dipole or multipole moment as a result of a separation of electric charge. In the context of contradiction, the answer is absolutely not! BF3 is not polarised. Electronegativity differences of less than 0.5 are considered nonpolar in the majority of cases.

Summary
On the periodic table, boron trifluoride (BTF) is the least electronegative element. SP2 hybridizations, such as BF3, are just one type of hybridization that can be used. Because the SP2 molecule contains only three boron atoms, a single bond is required to create a double bond between them.
Key Points to Consider When drawing The BF3 Molecular Geometry
The BF3 molecular can be drawn using a three-step process. Sketch the molecular geometry of the BF3 molecule, calculate the electron lone pairs in the central boron atom, then calculate the hybridization of the BF3 molecule, and finally give precise notation for the BF3 molecular geometry.
In the BF3 molecular geometry diagram, the number of valence electrons and the number of pairs of bond electrons are shown. A molecular hybridization theory and the Valence Shell Electron Pair Repulsion Theory can both predict the BF3 molecule’s geometry.
The VSEPR theory states that molecules will choose the BF3 geometrical shape that is most distant from one another within the specific molecular structure.
In addition, you must consider the polarities of the B-F bond in order to calculate the bond’s strength (dipole moment properties of the BF3 molecular geometry).
Since all (B-F) bonds have the same size and polarity, the bond dipole moment of the BF3 molecule, which is opposed to each other in the trigonal planar geometry, means that the BF3 molecule is classified as a polar molecule because of the polarisation of the boron-fluorine bonds toward fluorine’s more electronegative value.
The boron trifluoride molecule (BF3 molecular geometry) has a bond angle of 120 degrees between the F-B-F atoms. The electronegativity values of boron and fluorine atoms are different, with fluorine’s pull on the electron cloud being greater than boron’s. In the trigonal planar geometry, the bond polarity of B and F was cancelled against each other.
Consequently, it does not have a molecular structure with a permanent dipole moment. Due to an equal distribution of negative and positive charges, the BF3 molecule lacks a dipole moment.
| Geometry | Type | # of Electron Pairs | Ideal Bond Angle | Examples |
|---|---|---|---|---|
| linear | AB2 | 2 | 180° | BeCl2 |
| trigonal planar | AB3 | 3 | 120° | BF3 |
| tetrahedral | AB4 | 4 | 109.5° | CH4 |
| trigonal bipyramidal | AB5 | 5 | 90°, 120° | PCI5 |
| octohedral | ABG | 6 | 90° | SF6 |
| bent | AB2E | 3 | 120° (119°) | SO2 |
| trigonal pyramidal | AB3E | 4 | 109.5° (107.5°) | NH3 |
| bent | AB2E2 | 4 | 109.5° (104.5°) | H2O |
| seesaw | AB4E | 5 | 180°,120° (173.1°,101.6°) | SF4 |
| T-shape | AB3E2 | 5 | 90°,180° (87.5°/<180°) | CIF3 |
| linear | AB2E3 | 5 | 180° | XeF2 |
| square pyramidal | ABSE | 6 | 90° (84.8°) | BrFs |
| square planar | AB4E2 | 6 | 90° | XeF4 |
In short
The bond angle between the F-B-F atoms in the boron trifluoride molecule (BF3 molecular geometry) is 120 degrees. The BF3 molecule lacks a dipole moment because of an even distribution of negative and positive charges.
Advantages of BF₃
-
The North American compressed BF3 gas market is dominated by Gulbrandsen.
-
With quick delivery and superior logistics, Texas and LaPorte, where we have supply points, are ideally situated to meet the needs of the Gulf Coast region of the United States.
-
We can ship BF3 in bulk to any part of the world using our bulk tube trailers, which are built for international use.
Packaging of BF₃
We have a fleet of US-DOT approved containers capable of transporting BF3 with service pressures of approximately 1,800 PSIG: 840 kg/1,850 lbs of weight can be carried by spheres. Up to 10 MT (22,000 lbs) can be transported in Bulk Tube Trailers.
Additionally, BF3 is an example of how Gulbrandsen can lower the cost of chemical supply solutions by implementing logistics and manufacturing innovations. When Gulbrandsen pioneered the use of US-DOT approved ISO chassis and frames to ship BF3 as ocean cargo, it made Gulbrandsen an attractive and possible supplier of bulk BF3.
Experimental Properties of BF₃
When heated with alkaline earth metals or alkali metals, except magnesium, it emits dense, white fumes. Nitric acid (HNO3.2BF3) can be easily formed into coordination complexes with molecules that have only one unshared pair of electrons, which polymerizes unsaturated molecules.
Boron trifluoride, whether in liquid or gaseous form, has no reaction with chromium or mercury, even under extremely high pressures and for extended periods of time. This material’s formation enthalpy measures 1136.9 kJ/mol at 25 °C (as gas). It takes 4.20kJ/mol of heat to fuse one mole of hydrogen to one mole of oxygen at -126°C.
Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
A compound’s formula is not directly linked to its molecule’s shape. The valence-shell electron-pair repulsion (VSEPR) theory, developed about 30 years ago, predicts the shapes of these molecules based on their Lewis structures.
It’s based on the VSEPR theory, which states that the arrangement of electrons in the valence shell of a given atom should be minimised. The five compounds illustrate how the VSEPR theory can be applied to simple molecules.
An electron can be found only in two places in BeF2’s valence shell, one on either side of the central atom. Arranging these electron pairs so that they face in opposite directions reduces the attraction between them. Thus, according to the VSEPR theory, BeF2 should be a linear molecule with two Be-F bonds at a 180-degree angle.
On the central atom of boron trifluoride (BF3), valence electrons can be found in three places. Arranging these electrons in an equilateral triangle’s corners reduces their repulsion. As a result, the VSEPR theory predicts that the BF3 molecule will have a trigonal planar geometry with an F-B-F bond angle of 120o.
Atoms in BeF2 and BF3 are on the same plane, making them two-dimensional molecules. If the same restriction was applied to it, CH4 would yield a square-planar geometry with an H-C-H bond angle of 90o. When this system is allowed to expand into three dimensions, however, the H-C-H bond angle is 109o28’ in the resulting tetrahedral molecule.

PF5’s phosphorus atom’s valence electron repulsion can be reduced by distributing the electrons to the corners of a trigonal bipyramid, which has five pairs. Equatorial bipyramids have three positions labelled “equatorial” because they are located near the molecule’s centre.
In contrast, the other two are axial in that they are perpendicular to the plane of the equator. Three of the equatorial positions are at an angle of 120 degrees to each other, while one of them is at an angle of 90 degrees to the Earth’s surface.
Valence electrons can be found in six locations on the central atom of SF6. Electron repulsion can be reduced by distributing these electrons toward the octohadron’s corners. We’re more interested in the octahedron’s six vertices than its eight sides. In order to visualise the structure of an SF6 molecule, locate fluorine atoms on the X, Y, and Z axes of an XYZ coordinate system on opposite sides of sulphur.
Summarize
The Lewis structures of these molecules are used by the VSEPR theory to predict their shapes. The electron configuration in the valence shell of an atom should be minimised, according to this rule. Compounds like the ones shown here demonstrate the theory’s applicability to even the simplest molecules.
Incorporating Double and Triple Bonds into the VSEPR Theory
For compounds containing multiple bonds, the geometry around the atom is determined by how many electrons are available in the valence shell of an atom, not how many pairs of electrons are available.
The carbon atom in CO2 has four pairs of bonding electrons, but there are only two locations where these electrons can be discovered. (The C=O double bond on the left and the C=O double bond on the right have electrons in them.) Electron repulsion between the two C=O double bonds is reduced when they are situated in opposition to one another on the carbon atom. Because CO2 is a linear molecule, the VSEPR theory predicts that it will have a bond angle of 180o.
Additionally, the Lewis structure of the Carbinatecarbonate ion shows that the central atom has four pairs of valence electrons. Two C-O single bonds and one C-O double bond contain most of these electrons. When the three oxygen atoms are positioned toward the corners of an equilateral triangle, the repulsions between their electrons are reduced. CO32-should have a trigonal-planar geometry just like BF3, with an angle of 120o.
To summarize
The number of electrons in an atom’s valence shell determines the shape of the atom’s valence shell around the atom. In CO2, there are four pairs of bonding electrons, but only two places where they can be found. ’
The Role of Nonbonding Electrons in the VSEPR Theory
Valence electrons on both NH3 and H2O should be distributed toward each of the tetrahedron’s four corners, as depicted in the following figure. Predicting the distribution of valence electrons isn’t our goal. The goal is to predict the molecule’s structure based on this distribution of electrons. Both have been the same until now. Once nonbonding electrons are included, this no longer holds true.
The valence electrons on the central atoms of ammonia and water are predicted to point toward the corners of a tetrahedron by the VSEPR theory. This prediction cannot be directly tested due to our inability to precisely locate the nonbonding electrons. Predictions of the nuclei’s locations in these molecules can be tested experimentally based on the VSEPR theory’s results.
Using the positions of the nuclei in ammonia, we predict that the NH3 molecule has a trigonal pyramidal shape with nitrogen at the top. When it comes to the shape of water, however, it should have an angular or bent appearance. These predictions have been proven correct, reinforcing our confidence in the VSEPR theory.
Sum Up
NH3 and H2O should have their valence electrons distributed to the four corners of the tetrahedron. This is no longer the case when nonbonding electrons are included. Predictions based on VSEPR theory can be tested experimentally to see if they are correct.
Frequently Asked Questions
Following are some frequently asked questions related to BF3 molecular shape.
1. Is BF3 Vsepr shape?
The boron trifluoride molecule (BF3 molecular geometry) is tilted at a bond angle of 120 degrees between F-B-F. In the trigonal planar geometry, the bond polarity of B and F was cancelled against each other.
2. Is BF3 tetrahedral?
Because the octet of the central B atom in BF3 is incomplete, the compound has a tetrahedral structure.
3. Is BF3 molecule polar?
Molecules such as boric trifluoride (BF3) and ammonia (NH3) are polar, while BF3 is nonpolar.
4. Is BF3 trigonal planar or tetrahedral?
Trigonal Planar is the name given to the molecule’s geometry. The term “trigonometric planar” refers to a model in which three atoms surround a single atom in the centre. All three atoms are identical and form an equilateral triangle because of the 120° bond angles on each of them.
5. What is the molecular and electron geometry of BF3?
The trigonal planar shape is defined by the VSEPR theory as a structure that has three bonding pairs and no lone pairs. Because of this, BF3B F3 has trigonal planar electronic and molecular geometry.
6. Is BrF3 polar or nonpolar?
BrF3 (bromine trifluoride) is a polar molecule with a deformed or twisted trigonal bipyramidal structure because of two lone pairs on the central bromine atom. The BrF3 molecule is polar in nature because of the non-uniformity of its charge distribution on its atoms.
7. Why does a Bf3 molecule have a trigonal planar shape while an NH3 molecule is a trigonal pyramidal?
Because B has three electrons in its outermost shell while N has five, Bf3 has a planar shape while NH3 has a pyramidal shape. Because of the repulsion between the lone pair and the bond pair, NH3 has a pyramidal shape due to the presence of a lone pair of electrons.
8. What is the molecular shape of bi3?
Boron triiodide has the chemical formula BI3 and is a boron-iodine compound. Its molecular structure is trigonometrically planar.
9. Is BF3 ionic or covalent?
While covalent, the B-F bonds in this compound are extremely polar, resulting in an overall ionic character.
10. How is bf3 a nucleophile?
The extremely electronegative fluorine atoms covalently bound to the boron create a strong partial positive charge on the boron. As a result, BF3 is a potent Lewis acid and an electrophile because of its strong partial positive character.
Conclusion
For molecules with lone pairs, VSEPR theory sets up a graph that lists down the molecular geometry details. As opposed to the terminal atom, the central atom has the lowest electronegativity in the Lewis structure.
Atomic numbers are useful in identifying which of an atom’s valence electrons are located in the outermost shell. Net dipole moment and electronegativity difference less than 0.5 cancel each other out. Thus, the molecule has no polarity.
Related Articles
Nf3 Molecular Geometry
Ammonia Molecular Geometry