What is the P50? P50 may be a shorthand representation of hemoglobin-oxygen affinity. A lower P50 is protective in ambient hypoxemia, whereas increasing the p50 should be beneficial in hypoxia because of lung disease, anemia, and tissue ischemia. This is often because hemoglobin-oxygen affinity has complex effects on tissue oxygenation.

What is P50 Hemoglobin?
![]() The oxyhemoglobin dissociation curve shows the connection between the hemoglobin saturation (SO2) at different oxygen tensions (PO2).
The oxyhemoglobin dissociation curve shows the connection between the hemoglobin saturation (SO2) at different oxygen tensions (PO2).
![]() The p50 is the oxygen tension at which hemoglobin is 50% saturated. The traditional P50 is 26.7 mm Hg.
The p50 is the oxygen tension at which hemoglobin is 50% saturated. The traditional P50 is 26.7 mm Hg.
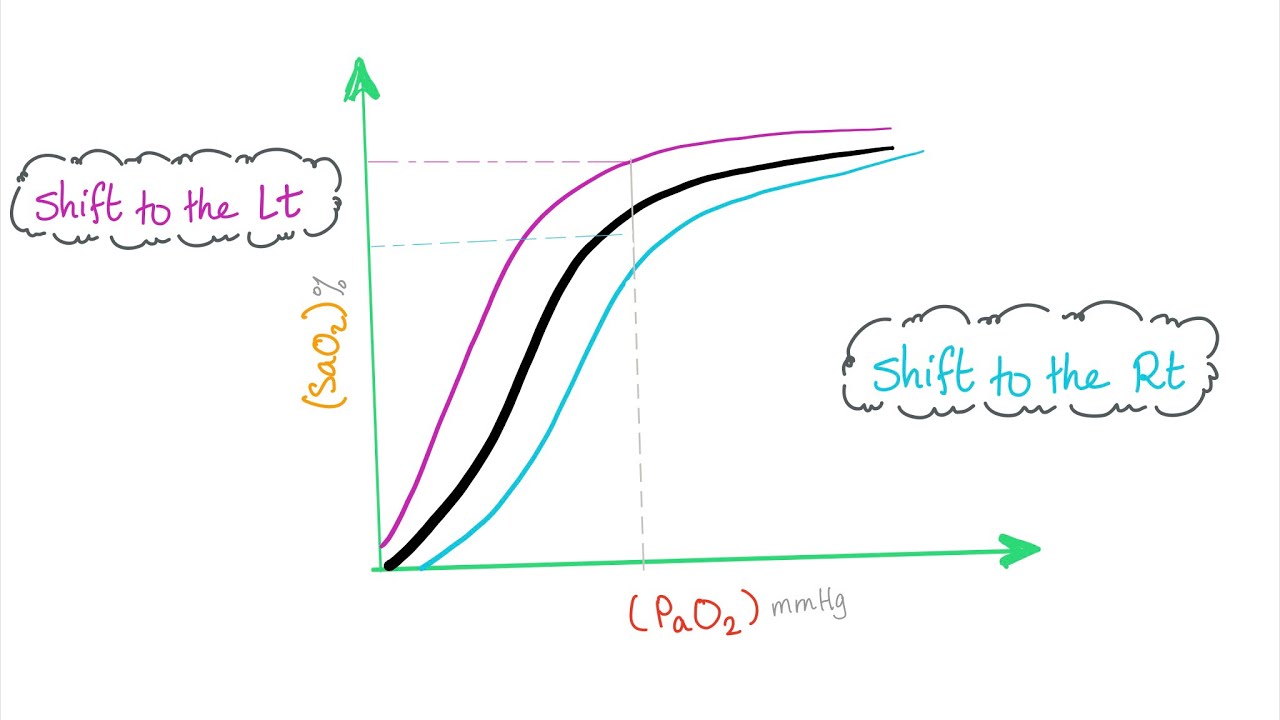
![]() Shifting the curve to the left or right has little effect on the SO2 within the normal range where the curve is fairly horizontal; away the greater effect is seen for values on the steeper part of the curve.
Shifting the curve to the left or right has little effect on the SO2 within the normal range where the curve is fairly horizontal; away the greater effect is seen for values on the steeper part of the curve.
Shifting of the oxyhemoglobin dissociation curve
![]() A rightward shift increases p50 and lowers hemoglobin’s affinity for O2.
A rightward shift increases p50 and lowers hemoglobin’s affinity for O2.
![]() A leftward shift decreases p50 and increases hemoglobin’s affinity for O2.
A leftward shift decreases p50 and increases hemoglobin’s affinity for O2.
Factors affecting the oxyhemoglobin dissociation curve
Hemoglobin Dissociation Curve
-
A decrease in pH shifts the quality curve to the proper, while a rise shifts it to the left. This is often referred to as the Bohr effect.
-
CO2 affects the curve in two ways: first, it influences intracellular pH, referred to as the Bohr Effect, which is physiologically far more important), and second, CO2 accumulation causes carbamino compounds to be generated through chemical interactions.
-
Increasing CO2 has the effect of shifting the curve to the proper and decreasing shifts the curve to the left.
-
2,3-diphosphoglycerate is made in erythrocytes during glycolysis. the assembly of two,3-DPG is probably going a crucial adaptive mechanism, because the assembly increases for several conditions within the presence of diminished peripheral tissue O2 availability, like hypoxemia, chronic lung disease, anemia, and congestive coronary failure, among others.
-
High levels of two,3-DPG shift the curve to the proper, while low levels of two,3-DPG cause a leftward shift, seen in states like septic shock and hypophosphatemia.
-
Temperature doesn’t have so dramatic effect because hyperthermia causes a rightward shift and hypothermia causes a leftward shift
-
 Hemoglobin binds with carbon monoxide gas 240 times more readily than with oxygen, and thus the presence of carbon monoxide gas can interfere with the hemoglobin’s acquisition of oxygen. additionally to lowering the potential for hemoglobin to bind to oxygen, carbon monoxide gas also has the effect of shifting the curve to the left.
Hemoglobin binds with carbon monoxide gas 240 times more readily than with oxygen, and thus the presence of carbon monoxide gas can interfere with the hemoglobin’s acquisition of oxygen. additionally to lowering the potential for hemoglobin to bind to oxygen, carbon monoxide gas also has the effect of shifting the curve to the left. -
With an increased level of carbon monoxide gas, an individual can suffer from severe hypoxia while maintaining a traditional PO2.
-
Methemoglobinemia causes a leftward shift within the curve.
Oxyhemoglobin Dissociation Curve
- Normal P50: 27 torr
- Right Shift: acidemia and hypercarbia (Bohr effect), increased temperature, 2,3-DPG.
- Left Shift: alkalosis, hypocarbia, decreased temperature, carbon monoxide, methemoglobin, hypophosphatemia (critical care patients).
Summary: The oxygen dissociation curve plots the half of saturation against the partial pressure of oxygen, and its contribution to the entire oxygen content. This is often an S shaped curve thanks to the alterations in hemoglobin’s affinity for oxygen in response to other physiologic factors. Please note the line at rock bottom of the graph. This represents the dissolved O2. Dissolved O2 may be a linear relationship to its partial pressure and leads to a line .
What is Myoglobin P50?
Myoglobin may be a small monomeric haem protein found in striated muscle and myocardium
![]() It’s synthesized locally.
It’s synthesized locally.
![]() Its synthesis is assumed to be stimulated by hypoxia.
Its synthesis is assumed to be stimulated by hypoxia.
![]() It’s degraded in muscles.
It’s degraded in muscles.
![]() Circulating myoglobin is rapidly faraway from the bloodstream, probably by the spleen and liver.
Circulating myoglobin is rapidly faraway from the bloodstream, probably by the spleen and liver.
![]() It contains one O2 binding site.
It contains one O2 binding site.
![]() The oxygen-myoglobin dissociation curve is hyperbolic instead of sigmoid.
The oxygen-myoglobin dissociation curve is hyperbolic instead of sigmoid.
![]() This is often because the hemoglobin molecule may be a tetramer with positive cooperativity between oxygen binding sites, which changes the form of its oxygen dissociation curve.
This is often because the hemoglobin molecule may be a tetramer with positive cooperativity between oxygen binding sites, which changes the form of its oxygen dissociation curve.
![]() Myoglobin features a very high affinity for oxygen: the p50 is ~ 2.7 mmHg.
Myoglobin features a very high affinity for oxygen: the p50 is ~ 2.7 mmHg.
The physiological relevance of this is:
- Within the tissues, PO2 is low.
- Hemoglobin has a low affinity for oxygen at this PO2, and it releases bound oxygen into the tissue fluids.
- Myoglobin features a high oxygen affinity at this PO2, and it collects the released oxygen.
- During this fashion, oxygen is transferred between hemoglobin and myoglobin.
- The most role of myoglobin is to take care of the oxygen supply to exercising muscle.
- The entire oxygen store of myoglobin within the physical body is around 200-300ml, like about 7-10 seconds of muscle activity.
What is P50 myoglobin in mmhg?
![]() Around 2.5-3.0 mmHg
Around 2.5-3.0 mmHg
- Role of myoglobin in oxygen storage. Note that the p50 value is somewhere around 2.5-3.0 mmHg. This protein binds oxygen with a really high affinity, and is fully saturated at a desperately low PO2, which probably represents the PO2 of muscle fibres at rest.
How to calculate P50?
Abstract
![]() Background: The hemoglobin-oxygen affinity is conveniently described because of the oxygen tension at which the hemoglobin is 50% saturated (p50). We used two compared methods of single-point analysis for p50 calculation by using clinical data.
Background: The hemoglobin-oxygen affinity is conveniently described because of the oxygen tension at which the hemoglobin is 50% saturated (p50). We used two compared methods of single-point analysis for p50 calculation by using clinical data.
![]() Methods: From patients submitted to anesthesia for operation, 114 arterial or blood samples were analyzed by using the Sigaard-Andersen oxygen status algorithm (p50OSA) and Doyle’s method (p50Doyle) supported Hill’s equation.
Methods: From patients submitted to anesthesia for operation, 114 arterial or blood samples were analyzed by using the Sigaard-Andersen oxygen status algorithm (p50OSA) and Doyle’s method (p50Doyle) supported Hill’s equation.
![]() Results: The O2 saturation and tension varied respectively between 0.640-0.960 and 3.80 kPa-11.00 kPa. The bland-Altman analysis showed a mean difference of 0.04 kPa (SD 0.12 kPa). the bounds of the agreement were -0.20 kPa and +0.28 kPa.
Results: The O2 saturation and tension varied respectively between 0.640-0.960 and 3.80 kPa-11.00 kPa. The bland-Altman analysis showed a mean difference of 0.04 kPa (SD 0.12 kPa). the bounds of the agreement were -0.20 kPa and +0.28 kPa.
![]() Conclusions: The Siggaard-Andersen oxygen status algorithm is presently the foremost clinically useful single-point method of p50 calculation.
Conclusions: The Siggaard-Andersen oxygen status algorithm is presently the foremost clinically useful single-point method of p50 calculation.
P50 of fetal hemoglobin
![]() Fetal hemoglobin (HbF) is that the dominant sort of hemoglobin present within the fetus during gestation. HbF is produced by erythroid precursor cells from 10 to 12 weeks of pregnancy through the primary six months of postnatal life. HbF contains two alpha and two gamma subunits, while the main sort of adult hemoglobin, hemoglobin A (HbA), contains two alpha and two beta subunits.
Fetal hemoglobin (HbF) is that the dominant sort of hemoglobin present within the fetus during gestation. HbF is produced by erythroid precursor cells from 10 to 12 weeks of pregnancy through the primary six months of postnatal life. HbF contains two alpha and two gamma subunits, while the main sort of adult hemoglobin, hemoglobin A (HbA), contains two alpha and two beta subunits.
![]() The genes that express gamma chain proteins are present within the beta chain locus on chromosome 11. The gamma subunit differs from its adult counterpart therein it contains either an alanine or a glycine at position 136, both of which are neutral, nonpolar amino acids.
The genes that express gamma chain proteins are present within the beta chain locus on chromosome 11. The gamma subunit differs from its adult counterpart therein it contains either an alanine or a glycine at position 136, both of which are neutral, nonpolar amino acids.
![]() This difference introduces conformational changes to the protein that provides rise to many physiological differences in oxygen delivery that are important within the fetal circulation
This difference introduces conformational changes to the protein that provides rise to many physiological differences in oxygen delivery that are important within the fetal circulation
Cellular
![]() Fetal hemoglobin features a vital role within the transport of oxygen from maternal to foetal circulation . Oxygen transfer from the maternal circulation to the foetal circulation is formed possible by HbF having a high oxygen affinity but decreased affinity to 2,3-bisphosphoglycerate relative to HbA. The HbF oxygen dissociation curve is left-shifted as compared to HbA.
Fetal hemoglobin features a vital role within the transport of oxygen from maternal to foetal circulation . Oxygen transfer from the maternal circulation to the foetal circulation is formed possible by HbF having a high oxygen affinity but decreased affinity to 2,3-bisphosphoglycerate relative to HbA. The HbF oxygen dissociation curve is left-shifted as compared to HbA.
![]() The partial pressure at which HbF is half saturated with oxygen (p50) is nineteen torr , compared to 27 torr for HbA. This value indicates that HbF features a high affinity for oxygen, giving HbF the power to bind oxygen more readily from the maternal circulation.
The partial pressure at which HbF is half saturated with oxygen (p50) is nineteen torr , compared to 27 torr for HbA. This value indicates that HbF features a high affinity for oxygen, giving HbF the power to bind oxygen more readily from the maternal circulation.
![]() HbF also shows a decreased affinity for two ,3-bisphosphoglycerate (2,3-DPG), a metabolic intermediate produced in tissues with high energy use (low ATP, high acid production). a better binding affinity to 2,3-DPG causes a right shift in HbA, favoring the unloading of oxygen. 2,3-DPG is important for correct oxygen unloading within the postnatal circulation. Another property of foetal circulation , allowing oxygen transfer to the fetus, is fetal hematocrit.
HbF also shows a decreased affinity for two ,3-bisphosphoglycerate (2,3-DPG), a metabolic intermediate produced in tissues with high energy use (low ATP, high acid production). a better binding affinity to 2,3-DPG causes a right shift in HbA, favoring the unloading of oxygen. 2,3-DPG is important for correct oxygen unloading within the postnatal circulation. Another property of foetal circulation , allowing oxygen transfer to the fetus, is fetal hematocrit.
Clinical Significance
![]() One medical application of the properties of HbF is within the management of red blood cell anemia. At baseline, HbF accounts for two to twenty of hemoglobin in red blood cell disease, counting on various patient-dependent factors, and this elevation appears to flow from to the greater oxygen affinity of HbF; therefore, HbF is a smaller amount likely to deoxygenate, sickle, and cause pain crises in these patients.
One medical application of the properties of HbF is within the management of red blood cell anemia. At baseline, HbF accounts for two to twenty of hemoglobin in red blood cell disease, counting on various patient-dependent factors, and this elevation appears to flow from to the greater oxygen affinity of HbF; therefore, HbF is a smaller amount likely to deoxygenate, sickle, and cause pain crises in these patients.
![]() Indeed, red blood cell disease patients don’t manifest symptoms in infancy thanks to elevated HbF, but as HbF decreases, patients may become symptomatic. HbA shows a decreased half-life in red blood cell disease because vaso-occlusive crises that occur during sickling and deoxygenation induce hemolysis. Through an unknown mechanism, the pharmacologic drug hydroxyurea increases the fraction of HbF found in adults.
Indeed, red blood cell disease patients don’t manifest symptoms in infancy thanks to elevated HbF, but as HbF decreases, patients may become symptomatic. HbA shows a decreased half-life in red blood cell disease because vaso-occlusive crises that occur during sickling and deoxygenation induce hemolysis. Through an unknown mechanism, the pharmacologic drug hydroxyurea increases the fraction of HbF found in adults.
![]() Treatment with hydroxyurea is indicated in patients that have frequent pain crises, acute chest syndrome, or severe anemia. By increasing HbF, hydroxyurea reduces the need for transfusions in patients with red blood cell anemia
Treatment with hydroxyurea is indicated in patients that have frequent pain crises, acute chest syndrome, or severe anemia. By increasing HbF, hydroxyurea reduces the need for transfusions in patients with red blood cell anemia

Hemoglobin p50 testing
![]() Hemoglobin-O2 Affinity (p50) Testing (Oxygen Dissociation, p50, Erythrocytes). Among the rare causes of polycythemia is hereditary polycythemia thanks to the presence of high O2 affinity hemoglobin. quite 100 such abnormal hemoglobins are described. they’re related to increased erythrocyte count, increased blood hemoglobin concentration, increased hematocrit (to values as high as 60%), but normal leukocyte and platelet counts, and no splenomegaly. a number of these hemoglobin variants are often detected by electrophoresis; many cannot.
Hemoglobin-O2 Affinity (p50) Testing (Oxygen Dissociation, p50, Erythrocytes). Among the rare causes of polycythemia is hereditary polycythemia thanks to the presence of high O2 affinity hemoglobin. quite 100 such abnormal hemoglobins are described. they’re related to increased erythrocyte count, increased blood hemoglobin concentration, increased hematocrit (to values as high as 60%), but normal leukocyte and platelet counts, and no splenomegaly. a number of these hemoglobin variants are often detected by electrophoresis; many cannot.
![]() However, the presence of a high O2 affinity hemoglobin variant in the blood can nearly always be detected by measurement of hemoglobin-O2 affinity. Congenital cyanosis could also be thanks to the presence of a coffee O2 affinity hemoglobin, and these can also be detected by the O2 affinity study.
However, the presence of a high O2 affinity hemoglobin variant in the blood can nearly always be detected by measurement of hemoglobin-O2 affinity. Congenital cyanosis could also be thanks to the presence of a coffee O2 affinity hemoglobin, and these can also be detected by the O2 affinity study.
![]() The hemoglobin-O2 affinity assay plots O2 saturation in percent on the ordinate vs. pO2 on the abscissa. the whole O2 affinity curve of hemoglobin is plotted from 0% to 100% saturation, yielding a smooth curve supported by many instantaneous measurements. From this, the pO2 is decided at which O2 saturation is 50%, and this is often the p50.
The hemoglobin-O2 affinity assay plots O2 saturation in percent on the ordinate vs. pO2 on the abscissa. the whole O2 affinity curve of hemoglobin is plotted from 0% to 100% saturation, yielding a smooth curve supported by many instantaneous measurements. From this, the pO2 is decided at which O2 saturation is 50%, and this is often the p50.
![]() Additionally, the curve is inspected to gauge whether it exhibits the traditional sigmoidicity since some high O2 affinity hemoglobin variants have nearly normal p50 but exhibit non-sigmoidal O2 affinity curves.
Additionally, the curve is inspected to gauge whether it exhibits the traditional sigmoidicity since some high O2 affinity hemoglobin variants have nearly normal p50 but exhibit non-sigmoidal O2 affinity curves.
P50 oxygen pregnancy
![]() The influence of tobacco smoke during pregnancy on Hb concentration and O2 affinity of smoker women has been studied. 59.0 non smoker (NSW) and 45.0 smoker women (SW) were investigated during pregnancy and early puerperium.
The influence of tobacco smoke during pregnancy on Hb concentration and O2 affinity of smoker women has been studied. 59.0 non smoker (NSW) and 45.0 smoker women (SW) were investigated during pregnancy and early puerperium.
![]() Results have shown: 1) a small non significant increase in oxygen affinity of SW; 2) a big decrease in oxygen affinity in SW at term and early puerperium, an equivalent as in NSW; 3) a big increase in haemoglobin concentration of SW at term as compared to NSW.
Results have shown: 1) a small non significant increase in oxygen affinity of SW; 2) a big decrease in oxygen affinity in SW at term and early puerperium, an equivalent as in NSW; 3) a big increase in haemoglobin concentration of SW at term as compared to NSW.
![]() The tiny modifications of oxygen affinity of SW and therefore the increase in haemoglobin concentration at term suggest that the oxygen delivery from the maternal to the fetal blood are probably unaffected by these factors.
The tiny modifications of oxygen affinity of SW and therefore the increase in haemoglobin concentration at term suggest that the oxygen delivery from the maternal to the fetal blood are probably unaffected by these factors.
Reference
 Frequently Asked Questions
Frequently Asked Questions
People are also wondering about following queries:
1- What does P50 do for your skin?
“Salicylic Acid makes the merchandise great for combating acne and keeping skin beyond blemishes,” Dr. Allouche said. “p50 also contains ingredients, like Vitamin B3, that improves the strength of the epidermis, balance the surface pH of the skin, all while enhancing epidermal renewal.”
2- How often do you have to use P50?
It is safe to use them twice each day , if your skin needs it. “How long do i want to attend to use the Lotions p50 if i exploit retinol?” a minimum of 2 weeks. “How often should I exfoliate?” The Lotions P50 are daily face exfoliators. they will even be used morning and evening if necessary, counting on your skin’s needs.
3- Which P50 lotion is best?
Lotion P50 1970 is suggested for greasy and acne prone skin instants. This version is ideal for your daily use and skincare routine. It helps to fight excess oil and deeply moisturizes at an equivalent time, provides excellent look after your skin.
Conclusion
![]() What is the P50?
What is the P50?
- The P50 represents the partial pressure at which hemoglobin is 50 percent saturated with oxygen.
- Normal P50 is 27 torr
- P50 provides a way of quantifying the hemoglobin’s affinity (willingness to bond) with oxygen. Reflects what are called shifts of the dissociation curve.
- Right shift – hemoglobin has decreased affinity, increased P50 – takes more oxygen to succeed in 50% (higher partial pressure to urge 50% saturated)
- Left shift – increased affinity, decreased P50 – less oxygen to succeed in 50% (less partial pressure to urge 50% saturated)
Related Articles
What is P4O10?
■■■■■■■■ analgesic IP 110
Stretch Mark Tattoos
Chin Filler
Hand-Tied Extensions
Laser Hair Removal Before and After