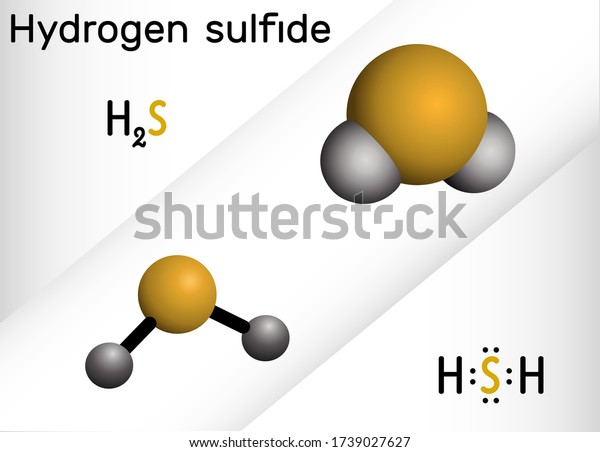
H2S Polar Or Nonpolar? Hydrogen sulfide is non-polar on account of its non-polar H – S bonds. The EN difference between hydrogen and sulfur is 0.4, so hydrogen and sulfur form non-polar bonds. Although it has an asymmetrical molecular figure, the entire patch is non-polar pretenses to the absence of any polar bonds.
Polar OR Non-Polar
H2S is the chemical formula for the emulsion hydrogen sulfide. Hydrogen sulfide is a covalent emulsion that’s composed out of 2 hydrogen tittles clicked to a central sulfur snippet.
Like water (H20), hydrogen sulfide is a hydrogen chalcogenide a emulsion made from hydrogen and a group 16 element (oxygen, sulfur, selenium, tellurium.
Hydrogen sulfide is non-polar on account of its nonpolar H – S bonds. The EN difference between hydrogen and sulfur is 0.4, so hydrogen and sulfur form non-polar bonds.
Although it has an asymmetrical molecular figure, the entire patch is non-polar pretenses to the absence of any polar bonds.
H2S- Hydrogen Sulphide:
| Compound Name | Hydrogen Sulphide | |
| Symbol/Formula | H2S | |
| Molar Mass | 34.1g/mol | |
| Smell | Foul odor | |
| Polar | No |
Hydrogen sulfide is most generally encountered as a product of the anaerobic respiration of sulfidogenic organisms. For case, some bacteria that operate in the absence of oxygen use sulfate ions (SO4 –) as the terminal electron acceptor during cellular respiration which reduces it into H2S.
In other words, sulfidogenic organisms breathe sulfur and exhale hydrogen sulfide. Again, in aerobic organisms, molecular oxygen (O2) acts as the terminal electron acceptor during respiration, which is reduced into H2O. It’s also the product of processes in tinderboxes and natural gas conformations.
Hydrogen sulfide is known for its pungent odor that’s described as rotting eggs. It’s combustive and will reply with heat and oxygen to produce sulfur dioxide (SO2) and water.
Summary:
Hydrogen sulfide is toxic to humans in large quantities. Its position of toxin is similar to that of carbon monoxide (CO). When gobbled, hydrogen sulfide will bind to enzymes in the mitochondria, which prevents cellular respiration.
Opposition
Basically, opposition in chemistry is a measure of how unevenly distributed electrons in a patch are. When two tittles form a covalent bond, they do so by participating valence electrons. Each element has an electronegativity which is a measure of how hard they pull on electrons.
When two rudiments that differ greatly in their electronegativity’s form a covalent bond, the further electronegative element will pull hard of the participated electrons than the lower electronegative element.
The result is that the participated electrons are pulled near to the further electronegative element. The uneven relegation of electric charges in the patch gives the more electronegative element a partial negative charge and the less electronegative element a partial positive charge.
This is what it means for a patch to be polar; it has a incompletely charged dipole across its structure on account of the uneven spatial distribution of electrons.
Whether or not two tittles will form a polar orn on-polar bond is dependent on those rudiments’ separate electronegativity’s. If two rudiments have an EN difference between0.5 and 2, the bond is generally considered polar.
If the difference is lower than 0.5, it’s considered functionally non-polar. Still, also the bond is fully polar, and is more duly appertained to as an ionic bond, If the difference is lesser than 2. For case, a patch of water is polar in virtue of its H-O bonds.
Hydrogen has an EN of 2.1 and oxygen has an EN value of3.5. the difference between these two values is1.4, so H-O bonds are considered polar, with a partial negative charge on the oxygen. Applying the former assignment on opposition, we can find out if hydrogen sulfide is a polar emulsion.
Hydrogen has an EN value of2.1 and sulfur has an EN value of2.5. the difference between these two values is lower than0.5, so H-S bonds are classified as non-polar.
Since hydrogen sulfide consists entirely of non-polar H-S bonds, the entire patch is non-polar. Rigorously speaking, H-S bonds aren’t fully non-polar. Sulfur is slightly more electronegative than hydrogen, so it does pull slightly tight on the participated electrons.
This opposition is veritably weak however, and virtually, it’s useful to treat veritably weakly polar bonds as if they aren’t polar at all. So Indeed though H-S bonds are technically a little bit polar, utmost of the time it’s safe to treat them as if they’renon-polar.
The only trulynon-polar bonds are formed between tittles with identical EN values (like the diatomic motes) The veritably slight opposition of hydrogen sulfide has significant goods at small scales, so in certain circumstances, it would be applicable to treat H-S bonds as polar.
Hydrogen sulfide is a triatomic (3- snippet) patch that consists of a central sulfur snippet and 2 terminal hydrogen tittles. Like a patch of water, hydrogen sulfide has a fraudulent geometric structure with a bond angle of92.1 ° and bond lengths of 136 picometers (1 picometer = 1 trillionth of a cadence).
It’s a bit thick than air and is explosive in the presence of oxygen and heat. Hydrogen sulfide is slightly answerable in water, and will separate into a lone proton (H) and a hydrosulfide ion (HS −). This geste makes hydrogen sulfide a weak acid.
Hydrogen sulfide is combustive and will reply with oxygen and heat to form sulfur dioxide and water. Under high temperature, sulfur dioxide will convert to essential sulfur and water, so the combustion of hydrogen sulfide is frequently used as one of the way to produce pure essential sulfur.
It reacts with essence ions to form essence sulfides, utmost generally with lead (Pb) to form lead (II) sulfide (PbS). Again, treating essence sulfides with a strong acid results in the product of hydrogen sulfide.
One of the primary natural sources of hydrogen sulfide is the exertion of sulfidogenic bacteria. Sulfidogenic bacteria use sulfur rather of oxygen for their metabolisms. During sulfidogenic respiration, bacteria will use sulfate ions as a reducing agent to carry electrons on the electron transport train.
At the end of this response, the sulfate ions are reduced into hydrogen sulfide which is released into the terrain. The exertion of sulfidogenic bacteria and their hydrogen sulfide products are responsible for the rotting smell associated with places with large amounts of decaying organic matter, like morasses or semasters.
The exertion of sulfidogenic bacteria is of pivotal significance to the sulfur cycle on earth. Therefore, hydrogen sulfide is one of the main ingredients of the sulfur cycle. The sulfur cycle is the process by which sulfur is cycled through the terrain, into living organisms, and back into the terrain.
SUMMARY:
Sulfur is a necessary trace element for living organisms, so the sulfur cycle is what keeps a constant force of essential sulfur for living organisms to use. The product of hydrogen sulfide by sulfidogenic bacteria represents an important step in this cycle; the product of the sulfur that will ultimately make its way into living organisms.
Geological Exertion
Small Quantities of hydrogen sulfide are also produced in geochemical responses in the Earth’s crust. The earth’s crust contains large amounts of sulfur and sulfur- containing minerals.
Under the presence of heat and pressure, essence sulfide composites will suffer hydrolysis with water to form a essence oxide and hydrogen sulfide gas.
As similar, hydrogen sulfide is a natural product of the process that creates natural gas. In fact, a large quantum of hydrogen sulfide is produced via the separation of it from natural gas deposits. Analogous mechanisms also affect in the conformation of hydrogen sulfide in thermal ocean reflections.
Although hydrogen sulfide is extremely poisonous to humans in large amounts, small quantities of hydrogen sulfide play a pivotal part in mortal biology.
Summary:
Hydrogen sulfide in the body frequently acts a signaling patch that regulates the quantum of ATP product during cellular respiration. Hydrogen sulfide also seems to be intertwined in the vasoconstriction of be@st blood vessels and the rate of seed germination in shops.
Toxin Of Hydrogen Sulfide
In general, hydrogen sulfide is veritably poisonous to obligate oxygen recesses. Its mechanisms of action are analogous to that of carbon monoxide. Hydrogen sulfide will bind to important enzymes and cofactors, precluding them from doing their job during cellular respiration.
Since hydrogen sulfide is naturally produced in the mortal body, the body does have mechanisms for removing hydrogen sulfide, though these mechanisms can be outpaced by a large enough cure.
The symptoms of hydrogen sulfide poisoning are analogous to those of carbon monoxide poisoning; fatigue, dizziness, incapability to concentrate, loss of memory, and perversity.
Though originally a pungent odor, the body snappily acclimates to the smell, which can make people ignorant of its presence. It’s slightly thick than air, so it has a tendency to accumulate near the bottom of inadequately voiced spaces. The mortal body can tolerate low attention of hydrogen sulfide for some time.
In high attention, inhalation of hydrogen sulfide can be incontinently fatal or beget serious brain damage. Historically, croakers have diagnosed extreme cases of hydrogen sulfide poisoning by placing a bobby coin in the victim’s fund.
Still, it’ll reply with the bobby coin in their fund, oxidizing it and turning it green, If the case has high amounts of hydrogen sulfide in their body. Hydrogen sulfide is a tintless patch with a chemical formula H2S. It’s toxic and has a foul odor like a rotten egg.
Summary
Hydrogen sulfide is known for its pungent odor that’s described as rotting eggs. It’s combustive and will reply with heat and oxygen to produce sulfur dioxide (SO2) and water. Hydrogen sulfide is toxic to humans in large quantities.
So, is H2S polar or nonpolar?
H2S is a slightly polar patch because of its fraudulent structured geometrical structure and the small difference between the electronegativity of Hydrogen (2.2) and Sulfur (2.58) that results in a non zero dipole moment.
It fluently reacts with essence ions to affect in essence sulfides. It’s dangerous and poisonous, especially for oxygen inhalers. Being a sharp, it destructs essence like bobby turning into green in color after the response.
It was discovered in the time 177 by a druggist named Carl Wilhelm Scheele. This gas is produced by mortal bodies and we uses it as a signaling patch.
Opposition is described as how electrons are distributed in the patch. It shows wherewith electrons are attracted and pulled by the most electronegative snippet. Let’s find it out by learning little information about the conception of electronegativity as it does count to opposition.
Electronegativity represents the capability of rudiments to attract electrons. Therefore rudiments that attract further electrons will be more electronegative. Electronegativity determines the distribution of electrons to find the opposition of a patch.
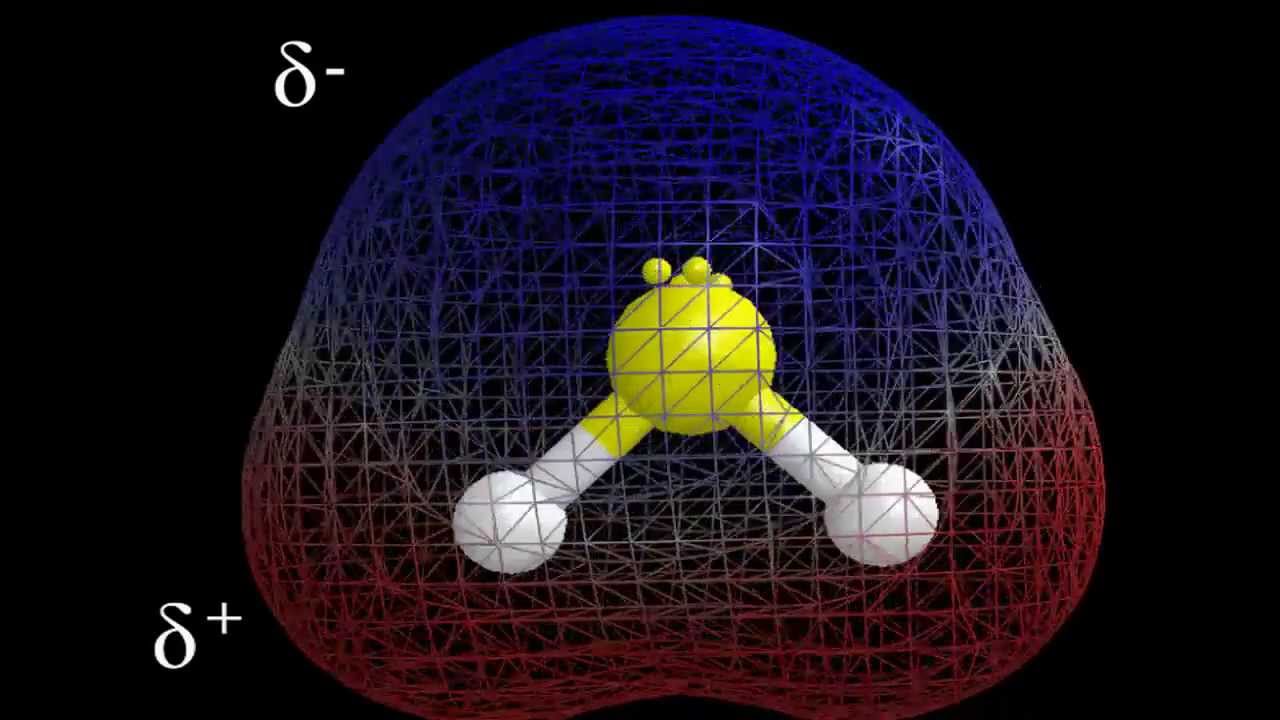
Since a patch is neutral but it’s called as concentrated when one side is more negative charge than the other positive- charged side. It has an asymmetrical arrangement of tittles, while there’s an uneven distribution of negative charges (electrons) outside the central snippet.
Water (H2O) is a polar patch because the further electronegative Oxygen has a advanced attention of electrons than the other snippet of the patch i.e. Hydrogen is appreciatively charged. You can check out the reason for the opposition of H2O.
Other motes like SO2, NH3,etc. are also polar motes. For nonpolar motes like CO2, you can check out the reason for thenon-polarity of CO2. The polar motes have an unstable sharing of electrons i.e. the charges aren’t balanced. But innon-polar motes, there are fairly equal figures of electrons.
Both tittles in the patch O2 have an equal viscosity of electronegativity, meaning they partake an equal number of electrons. Hence, the patch O2 isnon-polar. Motes that correspond of the same type of rudiments like H2, N2, Cl2,etc. are known as dipoles.
By dereliction, they’renon-polar motes. Hydrocarbons like methane (CH4), ethane (C2H6),etc. containing rudiments hydrogen and imitations are also nominated asnon-polar motes. Before you jump to find out the opposition of the patch, H2S, let’s talk about its bond opposition.
The opposition of a bond is calculated when the tittles of a patch have partial positive and negative charges. If the difference between the electronegativity’s of the two rudiments is lesser or equal to0.5, also the bond is polar.
With infinitesimal number 16, Sulfur pulls both the electrons of Hydrogen to complete its last shell and gains a negative charge. Hydrogen becomes positive- charged. Hence, the electronegativity of Sulfur becomes advanced than that of the Hydrogen snippet.
As you know, in the periodic table, electronegativity increases from left to right and decreases from top to down. The electronegativity of Hydrogen and Sulfur is 2.20 and 2.58, independently. Their electronegativity difference, 0.38, is lower than 0.5. Therefore, H2S is a non-polar bond.
Due to Sulfur being more electronegative than Hydrogen, it’s incompletely negative. As a result, it creates a dipole moment. To determine the opposition of any patch like H2S, it’s inversely important to find out its outside tittles, and shape.
There are two lone dyads of electrons on the central snippet Sulfur that causes the H-S bond to be in a fraudulent shape. Hence, the patch has an odd distribution of tittles around the central snippet making it non-symmetrical.
Because of its fraudulent shape, the dipole moment is created between the H-S bonds. The lesser the separation of charges more is the dipole moment between the tittles. Hence, Sulfur attracts further electrons and earnings a partial negative charge.
Hydrogen is a partial positive charge as it’s now left with smaller positive charges. Since the dipole moment has a direction and magnitude, it’s a vector volume. It directs towards further electronegative snippet.
When the arrows don’t cancel out each other, the patch becomes polar. The factor dipole of a patch shows the position of its opposition. Greater the opposition of a patch more is the value of its dipole moment. It can be also be defined as the product of charges of two tittles and the distance between them.
D = Q * R
D = dipole moment
Q = charge on tittles
R = distance between them
It’s used to produce hydrogen and sulfuric acid. It’s extensively used industrially to produce different kinds of inorganic composites. It’s used for manufacturing fungicides for crops on a larger scale. Hydrogen sulfide has its great use as heavy water used in nuclear power shops.
To calculate the opposition of any patch, certain factors need to be considered before you reach out to a conclusion. H2S is the polar patch with Hydrogen tittles clicked outside the central Sulfur snippet. It has an asymmetrical fraudulent shape that creates a dipole moment between tittles.
Sulfur is more electronegative than Hydrogen. This refers to Sulfur has further electrons than the ultimate bone. As you know the electronegative difference of the patch H2S is 0.4, which is considered to be negligible and has weak opposition too.
Technically, due to the absence of enough opposition between the tittles, the H2S is said to be anon-polar patch. This is an exceptional case that needs to be counted. According to certain studies, for a patch to be polar, the electronegativity has to be between0.5 and 2.
To know specifically about the electronic structure of H2S, you must also read an composition on H2S Lewis structure, figure, hybridization. FAQs Q1. Name the composites that have a polar bond. A1. The composites that have polar bonds are Water (H2O), Ammonia (MH3), and Sulfur Dioxide (SO2).
Summary
H2S is non-polar. A dipole moment is represented by a technical arrow refocused out from a incompletely positive end to the incompletely negative side. In the case of motes having further than two tittles, there are two possibilities to do-
Frequently asked questions
Here are some of the Frequently asked questions related to the article H2S Polar or Non-Polar:
1. Is H2S a polar or nonpolar molecule?
Hydrogen sulfide is polar because of the presence of lone brace of electrons in Sulfur and the electronegativity difference between Sulfur and H tittles. There are eight valence electrons present in the patch of hydrogen sulfide. Hydrogen sulfide patch has an angular figure with anon-zero dipole moment.
2. What type of bond is H2S?
Hydrogen sulfide (H2S) is a covalent emulsion because the bond forms between two hydrogens and one sulfur are covalent in nature. The covalent bond is formed due to the sharing of an electron that occurs between hydrogen and sulfur tittles in order to complete their quintet shell and hence, attains stability.
3. Is a hydrogen sulfur bond polar?
Yes, The short interpretation Sulfur is more electronegative than hydrogen, so the H-S bond is polar with electron viscosity advanced on the sulfur snippet.
4. Why is H2O polar but not H2S?
Because of H-Bonding. In Water, the oxygen snippet is largely electronegative and can centralize (incompletely) the hydrogen tittles, therefore hydrogen-hydrogen bonds between the H2O motes can be formed creating a veritably high boiling point. In H2S those bonds do n’t live, because sulfur is much less electronegative.
Conclusion
H2S (hydrogen sulphide) is polar patch. As hydrogen is lower electronegativity of H is lower than S so it has a dipole moment, which makes it a polar patch. Odour-rotten egg. Plant-Its generally plant in tinderboxes as a natural gas. Its is largely toxic and causes skin conditions.