Why compounds of transition metals are coloured?
Colour in transition-series metal compounds is generally due to electronic transitions of two principal types:
- charge transfer transitions
- d-d transitions
An electron may jump from a predominantly ligand orbital to a predominantly metal orbital , giving rise to a ligand-to-metal charge-transfer (LMCT) transition. These can most easily occur when the metal is in a high oxidation state. For example, the colour of chromate, dichromate and permanganate ions is due to LMCT transitions. More about d-d transitions:
An electron jumps from one d-orbital to another. In complexes of the transition metals the d orbitals do not all have the same energy. The pattern of splitting of the d orbitals can be calculated using crystal field theory. If you want to know more you can look up here .Also:
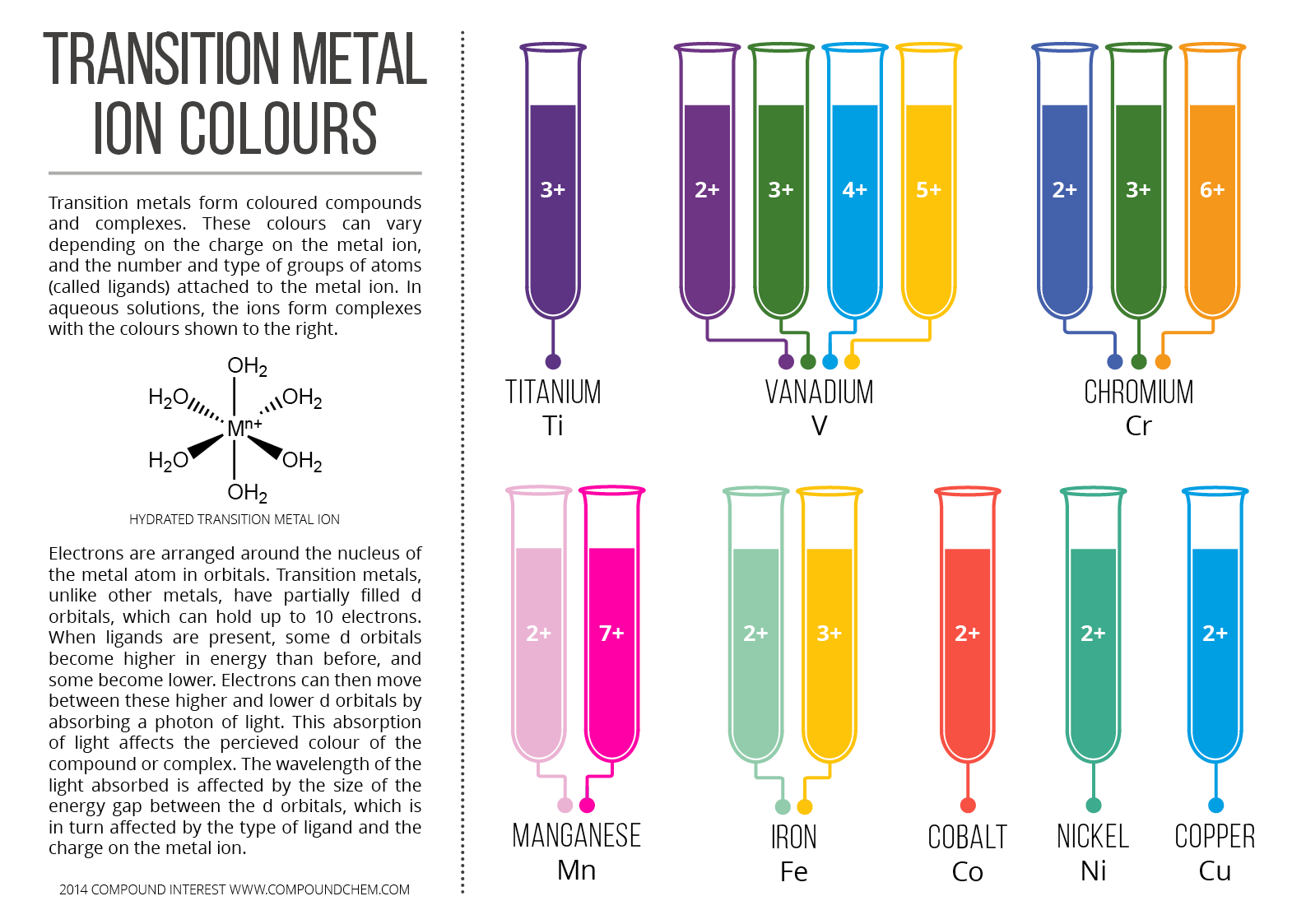 https://www.compoundchem.com/wp-content/uploads/2014/03/Transition-Metal-Ion-Colours-Aqueous-Complexes.png
https://www.compoundchem.com/wp-content/uploads/2014/03/Transition-Metal-Ion-Colours-Aqueous-Complexes.pngA simple explanation would be to know first what causes color. The key principle is electronic transition. To have an electronic transition, an electron must jump from a lower level to a higher level orbital. Now, light is energy right? So, when there is light, we see colors. But it doesnt stop there. The reason why transition metal in particular are colorful is because they have unfilled or either half filled d orbitals. There is Crystal field theory which explains the splitting of the d orbital, which splits the d orbital to a higher and lower orbital. Now, the electrons of the transition metal can jump. Note that light is absorb for electrons to jump, but this electrons will fall eventually back again to its ground state, releasing light of specific intensity and wavelength. We perceive this as colors.Now for the fun part. Note that electron cant transition if an orbital is already full. Take a look of Zinc in your periodic table. Note that a d orbital can only hold up to 10 electrons. Notice that zinc has 10 electrons in its d orbital. Yes, you guess it right, it will not color and is not consider a transition metal. zinc is not a transition metal but it is part of the d-block elements. Mind blown!