What is the name for P_2O_5?
The name is diphosphorus pentoxide.Binary compounds with oxygen in them have oxide as a last name.The first name is phosphorus.We give the numbers of each atom:di (=2) phosphorus penta (=5) oxide, which means: 2 phosphorus together with 5 oxygenThe a of penta is omitted when it comes before o as in oxide. Often the di- is also omitted (as the other compound also starts with di-), and it is called just phosphorus pentoxide.Extra :An older name would be phosphoric oxide, %P_2O_5%, as opposed to phosphorous oxide %P_2O_3%, which is the other possibility.The systematic name for %P_2O_5% is phosphorus(V) oxide. The numeral V signifies the oxidation number of phosphorus, which is +5.In the same way %P_2O_3% is called phosphorus(III) oxide, as the oxidation number of phosphorus is +3.
%P_2O_5% is commonly named phosphorus pentoxide. An interesting fact about phosphorus pentoxide is that %P_2O_5% is actually its empirical formula; its molecular formula is actually %P_4O_10%. The compounds name however was derived from its empirical formula, not from its molecular formula. The standard name for this compound is actually diphosphorus pentoxide. The di- prefix is used to show that the compound contains two phosphorus atoms and the penta- prefix is used to show it contains five oxygen atoms.
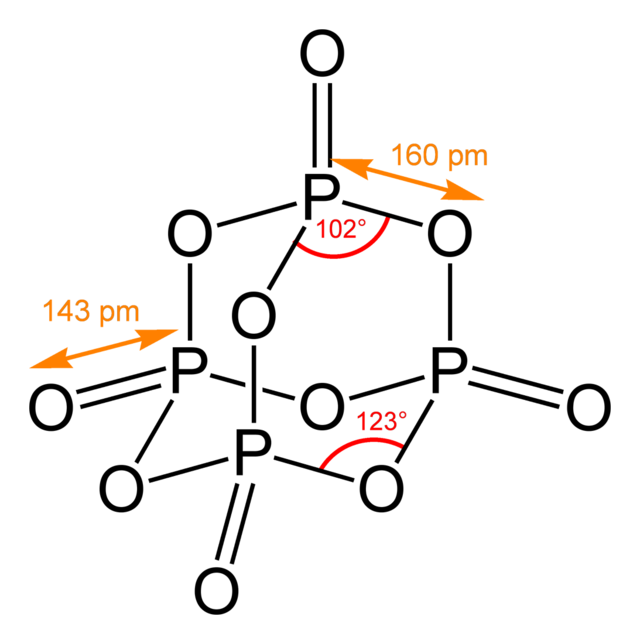
Phosphorus pentoxide is the most used name for %P_4O_10%, the white molecular solid whose empirical formula is %P_2O_5%. It is the anhydride of phosphoric acid. This is the image of the molecule from wikipedia.
 For its avidity of water, it is one of the most powerful known dehydrating agent.
For its avidity of water, it is one of the most powerful known dehydrating agent.