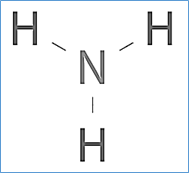
The Lewis structure of ammonia, NH3, is said to be three hydrogen atoms bonded to a nitrogen atom in the centre, with a single pair of electrons at the top of the atom.
Lewis structure of Ammonia (NH3)
If we want to make the Lewis structure of ammonia (NH 3 ) we will follow the following steps.
Start by drawing the general structure of the molecule by joining the hydrogen atoms with the nitrogen with single bonds:
Now we will proceed to count the total valence electrons of the hydrogen ( n H, val = 1) and nitrogen ( n N, val = 5) atoms , where “n” would be the valence of the atom:
3 H Valence + N Valence = 3 x 1 + 5 = 8
Then we have to calculate the number of electrons needed to completely fill the valence shells for hydrogen and nitrogen.
Hydrogen to complete its valence shell and therefore comply with the octet rule, it needs 2 electrons in its last orbital. But, since there are three hydrogen atoms, we will multiply these two electrons by three.
On the other hand, nitrogen needs 8 electrons in the last orbital to comply with the octet rule.
Now we do the sum:
3 H last layer + N last layer = 3 x 2 + 8 = 14
The next step will be to subtract the two results that we obtained and thus obtain the number of bonding electrons.
14 - 8 = 6 bonding electrons (three bonds).
Now if we look at the Lewis diagram of ammonia, we will see that we already have those 6 electrons (three bonds), therefore it already has all the necessary bonds.
There are 3 bonds and therefore 6 bonding electrons in the diagram.
Finally, fill in the unbonded electrons left in each atom. In total, there are 8-6 = 2 electrons left, these electrons will be added to the nitrogen.
In this way, we will finally obtain the Lewis structure of ammonia.
NH3 Hybridization – SP3
Ammonia, its molecular formula NH 3 and molecular model AB 3 E, its structure according to Lewis reveals that it has 3 bonding bonds with hydrogen (NH) and one non-bonding bond (NN) with Nitrogen itself.
Nitrogen has 5 electrons in its last level, as a central atom it has a hybridization of the Sp 3 type, with coordination number 4sp, with 1 e-free in three of its bonds (NH), and hydrogen has a single electron in its Also unique orbital (1s), the free electrons of Nitrogen agree with the electrons of Hydrogen by means of bonds type σ sp _σs (NH), to form Ammonia.
It is a polar molecule, the polarity is not cancelled, one part of the molecule accumulates a negative charge (δ - ), while the opposite part accumulates a positive charge (δ + ).
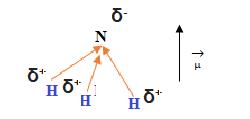
At first glance, Ammonia should have a tetrahedral geometry with an angle between the NH bond pairs of 109.5º, but in reality, this is not the case and it is explained by the electron pair repulsion theory of (the) valence shell ( TRPECV).
The pair of nonbonding electrons (NN), repels others links and away from it so that the angles between the bonding links (NH) is a smaller, and equal to 107 °, and its molecular geometry does not coincide with tetrahedral electronic geometry, but it will be trigonal pyramidal .
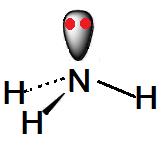
The ammonia molecule (NH3) has 3 NH single bonds and has a nonbonding electron pair in nitrogen. The molecular geometry is pyramidal.
NH3 connection angles
Bonding angle In NH3 the bonding angles are 107 degrees. It is a tetrahedral angle, which is 109.5 degrees. But it’s 107 degrees because the title pair takes up less space than the unbound pair.
The molecular form of NH3
The NH3 form is trigonal pyramidal. If there is one atom in the centre and three more in the corners, and all three molecules are equal, the molecular geometry takes the shape of a trigonal pyramid. Ammonia has this shape because nitrogen has 5 valence electrons and binds with 3 hydrogen atoms to complete the octet.
Is NH3 polar or non-polar?
Polar Nh3 The molecular geometry of NH3 is pyramidal trigonal with an asymmetric charge distribution on the central atom. Hence, this molecule is polar.
NH3 electronic geometry
We talked about almost everything about ammonia. Now let’s get to know the electron geometry. The electronic geometry of NH3 is “tetrahedral” because it has four groups of electrons. One group has a non-shared electron pair. “N” has tetrahedral electronic geometry. Thus, ammonia is an example of the molecule in which the central atom shared, as well as a pair of non-shared electrons.
Conclusion
So that’s it for ammonia. I hope I gave you the information about ammonia or NH3 that you expected. The geometry of molecules is an incredibly fascinating and exciting topic, and knowing these fundamentals is essential as you enter the realm of true chemistry. Always be curious and try to identify every aspect for you with the logic and magic of science.