H2S polaer or non polar, H2S is a some what polar particle because of the little contrast in electronegativity upsides of Hydrogen (2.2) and Sulfur (2.58) iotas. Hydrogen sulfide (H2S) is a tasteless gas with an impactful “spoiled egg” scent at low focuses.
Polarity of H2S
To comprehend the halfway polar nature of H2S, we want to comprehend its construction. The particle has two H molecules and a solitary S iota. Every H molecule has just a single electron, its valence electron.
H2S is a marginally polar particle because of the little distinction in electronegativity upsides of Hydrogen (2.2) and Sulfur (2.58) iotas. What’s more, the presence of two solitary matches on the contrary side of the two Hydrogen particles likewise makes the atom more polar and causes twisted shape mathematical construction of H2S.
Henceforth there are two valence electrons for the H iota (two H particles). Sulfur has six valence electrons. The table underneath gives insights regarding the electronic design of constituent iotas and their valence electrons.
| Molecule Name | Hydrogen di Sulfide | |
| Molar Mass | 34 g | |
| Reactions | Simple | |
| Color | White | |
| Test | Basic | |
| Atoms | Hydrogen and Sulfur |
The hybridization of the Hydrogen sulfide atom is sp3. The Sulfur molecule is in focus holding with two H particles shaping the bond point under 180 degrees. As per the VSEPR hypothesis, the solitary sets of electrons repulse one another.
Summary
There are two solitary sets of the sulfur molecule in the Hydrogen sulfide lewis structure. Likewise, Sulfur is attached to two H molecules. The aversion between the 2 solitary sets of electrons assumes a significant part in making its bowed atomic math. Consequently, the atom H2S is viewed as a polar particle.
Properties of Hydrogen sulfide
-
Hydrogen sulfide is a dreary poisonous gas. It exudes from sewers and as a result of current cycles. In low focuses, it smells like rotten eggs. The solvent is in Water and numerous different fluids. It structures hydro-sulfuric corrosive when disintegrated in Water.
-
It is profoundly dangerous and requires a grouping of 4% for a blazing fire when presented to a moderately cool hotness wellspring of 232 Degree Celsius. It is heavier and 1.136 occasions denser than air. This implies it will probably be found in low-lying regions with next to zero ventilation. Its edge of boiling over is – 60.4 degrees Celsius.
-
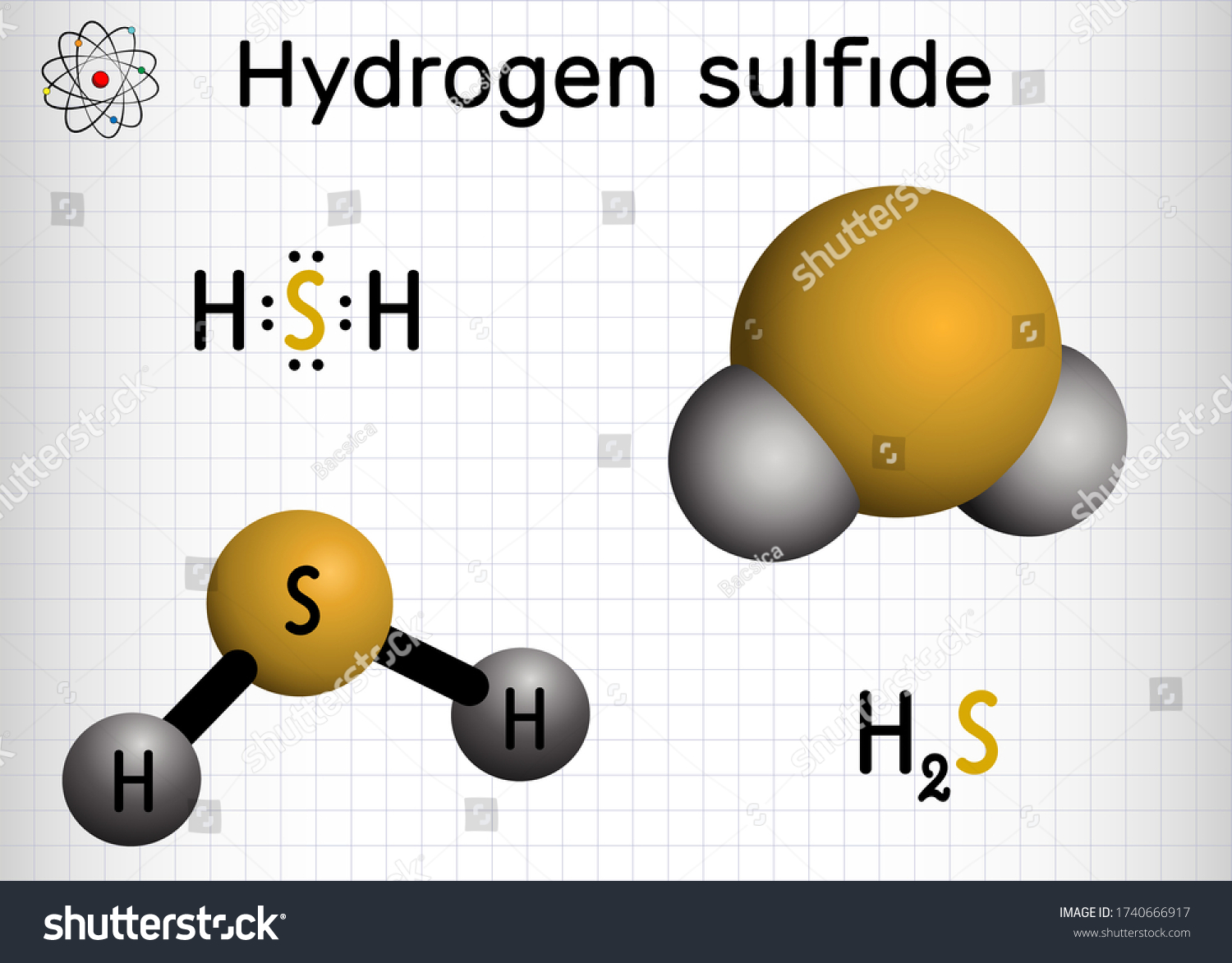
Two hydrogen molecules are situated on the two sides of the focal sulfur particle. Sulfur shares electrons with the adjoining hydrogen particles to make the atom stable. Two out of six valence electrons take an interest in the bond arrangement.
-
The excess four electrons are unbonded and are shown as specks close to the sulfur particle. These solitary sets of electrons make the H2S structure bowed.
What is Polarity?
The conveyance of electrical charge over the iotas joined by the bond causes extremity. In particular, it is observed that connections between particles of various components are electrically inequivalent. In hydrogen chloride, for example, the H particle is somewhat decidedly charged, while the Cl molecule is somewhat contrarily charged.
The slight electrical charges on disparate iotas are called fractional charges, and the presence of incomplete charges implies the event of polar security. A few instances of polar particles are Water (H2O), Ethanol, Ammonia and SO2 (Sulfur Dioxide).
The sub-atomic math of Hydrogen sulfide is polar; however, the bonds are not polar. Extremity is controlled by electronegativity. A particle is polar, assuming the construction of that atom isn’t symmetric. On account of symmetric construction, the dipole vectors on every particle drop one another, subsequent in the nonpolar idea of the atom. H2O is one more illustration of a polar particle.
The particle comprises two H iotas and one oxygen molecule. The distinction in electronegativity is 1.2 for every one of the H-O bonds. Since Oxygen is the more electronegative molecule, it applies a more noteworthy draw on the common electrons.
Oxygen additionally has two solitary sets of electrons. This fosters a dipole second in the water atoms. Carbon dioxide is a nonpolar atom that contains polar bonds. CO2 is a straight atom, and the C=O are polar bonds. The focal carbon has a net positive charge, while the two external oxygens have a negative charge.
H2S Lewis Structure
During the H2S Lewis structure, there are two hydrogen particles on the two sides of the focal sulfur molecule. There are eight valence electrons in the particle. The sulfur iota is in the construction, and valence electrons are organized around it.
Be that as it may, since the carbon dioxide particle is direct, these two bond dipoles counterbalance one another. Therefore, the general particle has no dipole. CO2 lewis structure shows CO2 has two oxygen molecules and one carbon iota fortified with a covalent bond.
Two Oxygen particles are situated on the sides of the carbon iota, where both these iotas share electrons and structure bonds. The carbon iota is the focal situation as it is the most un-electronegative molecule in the atom.
This applies an external power on the bond because of which the state of NH3 becomes unsymmetrical. Forerunner for natural Sulfur. It assumes a fundamental part in flagging pathways in the human body. Hydrogen sulfide is utilized basically to deliver sulfuric corrosive and Sulfur.
Ranchers use H2S as a farming sanitiser, and it is found in some cutting oils, which are coolants, and ointments planned explicitly for metalworking and machining processes, and different oils.
Hydrogen Sulfide Toxicity
Hydrogen sulfide is a profoundly poisonous gas and is the greatest reason for inhalational passings. Low-level openings to H2S might create nearby eye and mucous film bothering. Undeniable level openings might create deadly foundational harmfulness. Openings of 700-800 ppm of Hydrogen sulfide can cause loss of awareness.
Dipole moment
When two particles with various electronegative qualities cooperate, the electrons will quite often come nearer to the more electronegative iota. This development of electrons is addressed through the bond dipole second.
It is the result of the charge and the distance between the focuses of the positive and negative charges. It is indicated by the Greek letter ‘µ’. The presence of two solitary sets of electrons makes the atom twist.
Since Sulfur is more electronegative than Hydrogen, this makes the particle marginally polar. The vectorial amount of the bond dipole minutes in H2S produces a non-zero absolute dipole second. Thus, Hydrogen sulfide shows dipole-dipole connections.
Polarity
Hydrogen sulfide is polar due to the presence of solitary pair of electrons in Sulfur and the electronegativity distinction among Sulfur and H particles. There are eight valence electrons present in the particle of hydrogen sulfide.
Hydrogen sulfide particle has rakish math with a non-zero dipole second. H2S is an exceptionally poisonous gas and can be extremely hazardous whenever breathed in. Hydrogen sulfide is a lacklustre particle with a synthetic equation H2S. It is toxic and has a foul scent like a rotten egg.
All in all, is H2S polar or nonpolar? H2S is a somewhat polar particle due to its bowed moulded mathematical design and the little distinction between the electronegativity of Hydrogen (2.2) and Sulfur (2.58) that outcomes in a non zero dipole second.
It was found in 177 by a physicist named Carl Wilhelm Scheele. Human bodies deliver this gas, utilizing it as a flagging particle.
What do you mean by polarity?
Extremity is depicted as how electrons are conveyed in the particle. It shows wherewith electrons are drawn in and pulled by the most electronegative particle. We should think that it is out by learning little data about the idea of electronegativity as it makes a difference to extremity.
Electronegativity addresses the capacity of components to draw in electrons. Along these lines, components that draw in more electrons will be more electronegative. Electronegativity decides the circulation of electrons to track down the polarity of a particle.
Since a particle is unbiased yet spellbound, one side is more bad charge than the other positive-charged side. It has a topsy-turvy plan of particles, while there is a lopsided dispersion of negative charges (electrons) outside the focal iota.
Water (H2O) is a polar particle because the more electronegative Oxygen has a higher convergence of electrons than the other iota of the atom. For example, Hydrogen is charged. You can look at the justification behind the extremity of H2O.
Different atoms like SO2, NH3, and so forth are additionally polar particles. For nonpolar atoms like CO2, you can look at the justification for the non-extremity of CO2. The study of polar and nonpolar is characterized in the atom as how electrons are disseminated. This implies that the most electronegative molecule is drawn and pulled by electrons.
The extremity of Ammonia (NH3)
The extremity of the NH3 particle is because of the electronegativity contrast between N (3.04) and H ((2.2). This electronegativity contrast causes three dipole minutes a single way. This outcome in a net dipole second makes smelling salts a polar atom. Furthermore, the NH3 lewis structure shows a solitary pair of electrons present on Nitrogen.
We should discover, as extremity matters, by knowing little information about the standard of electronegativity. This is about polar and nonpolar. The propensity of components to draw in electrons is communicated by electronegativity. Consequently, there would be more electronegative parts that draw more electrons.
Since a particle is unbiased, it is alluded to as energized when one side is more negative than the other positive-charged side. It has a deviated iota structure, even though there is an unpredictable circulation past the focal molecule of negative charges (electrons).
The more electronegative Oxygen has a higher centralization of electrons than the other particle of the atom, i.e., Water (H2O) is a polar atom. Hydrogen is charged emphatically. Frequently known as H2S, squander gas, swamp gas, smell sodden, and harsh moist, hydrogen sulfide is a vapid gas known for its impactful ‘rotten egg’ scent at low focuses. It is incredibly explosive and truly harmful. Hydrogen sulfide is utilized or made in the scope of businesses, for example,
-
Refining oil and gas
-
Mining Sectors
-
Tanning
-
Handling mash and paper
-
Producing Rayon
Hydrogen sulfide likewise exists normally in sewers, dumps of waste, well water, oil and gas wells, and volcanoes. Hydrogen sulfide can aggregate in low-lying and fixed regions, like sewer vents, sewers, and underground phone vaults since it is heavier than air.
Problems by H2S
Its essence renders work possibly extremely dangerous in encased spaces. Hydrogen sulfide’s wellbeing impacts rely upon the amount H2S a specialist inhales and for how long. Likewise, at low focuses, notwithstanding, a few impacts are seen.
Impacts shift from moderate, disturbed migraines or eyes to intense, obviousness and demise. It is utilized for Hydrogen and sulfuric corrosive handling. It is usually utilized to handle different assortments of inorganic mixtures efficiently.
It is utilized on a greater scale to deliver pesticides for crops. The utilization of hydrogen sulfide as weighty Water in thermal energy stations is fine. Until you leap to the particle’s extremity, H2S, we should discuss its bond’s extremity.
The extremity of a bond is shaped when the iotas of a particle have preliminary positive and negative charges. Assuming the distinction between the two components’ electronegativity is more noteworthy than or equivalent to 0.5, then, at that point, the bond is polar. Positive-charged Hydrogen.
Consequently, Sulfur’s electronegativity becomes more prominent than that of the particle of Hydrogen. Electronegativity, as you most likely are aware, increments from left to squarely in the occasional table and diminishes from top to down.
Hydrogen and Sulfur have an electronegativity of 2.20 and 2.58, individually. Their distinction in electronegativity, 0.38, is more modest than 0.5. H2S is, in this way, a nonpolar bond. It is mostly negative because Sulfur is more electronegative than Hydrogen.
This creates a dipole second subsequently. Furthermore, a bolt that prompts a more electronegative iota addresses the dipole second. The dipole second from Hydrogen (delta +) to Sulfur is communicated by the H2S compound (delta-).
H-S bonds are rigorously talking, not nonpolar. Sulfur is somewhat more electronegative than Hydrogen; however, it pulls marginally harader on the shared electrons. In any case, this extremity is extremely frail, and it is essentially valuable to deal with exceptionally powerless polar bonds like they are not in the least polar.
Geometry of H2S
In this way, while H-S bonds are hypothetically somewhat polar, it is protected to regard them as nonpolar more often than not. The main genuinely nonpolar bonds are framed between molecules with indistinguishable EN esteems.
Hydrogen sulfide’s exceptionally slight extremity effects affect a limited scale, so treating H-S bonds as polar in certain circumstances would be sensible. Subsequently, while H-S bonds are hypothetically somewhat polar, it is protected to regard them as nonpolar more often than not.
Between iotas with indistinguishable EN esteems, the main nonpolar bonds are shaped. Hydrogen sulfide’s extremely slight effects affect a limited scale, so treating H-S bonds as polar in certain circumstances would be sensible.
It is similarly essential to discover the outer particles and structure to decide the extremity of any atom-like H2S. On the focal particle, Sulfur, two solitary sets of electrons permit the H-S to attach to be in a bowed structure.
The particle, hence, has unpredictable dissemination of iotas around the focal molecule, delivering it non-symmetric. The dipole second between the H-S securities is produced due to its bowed structure. The bigger the charge detachment, the more noteworthy the dipole second between the particles.
Sulfur subsequently draws more electrons and gets a negative incomplete charge. Hydrogen is presently left with less certain charges; Hydrogen is an incomplete positive charge. Since there are a course and the dipole second, it is a vector amount.
Electronegetivity
It focuses on more particles that are electronegative. Since the H2S particle isn’t balanced, there is a district of inconsistent sharing. The twisted shape implies that the top (where the solitary sets of electrons are) is more electronegative.
The Hydrogen molecules at the lower part of the construction are more certain. Hence, H2S is a polar H2S is a polar particle because of the presence of solitary pair electrons at the highest point of the atom, making a locale of a halfway bad charge due to electron-electron shock.
H2S has fundamentally the same design as H2O (see the lewis speck structure for H2O and the polar/nonpolar clarification at the connected addresses). Nonetheless, because of the bigger size of the sulfur molecule contrasted with Oxygen, the bond point (for example, the more modest point between the two hydrogen particles) is 92˚ contrasted with 107.5˚ for H2O.
Sulfur contains a lot more electrons, which eventually require significantly more space because of electron-electron aversion. The diminished electronegativity of Sulfur, when contrasted with Hydrogen (2.58 versus 2.20, individually), implies that the particle is substantially less polar, generally speaking, when contrasted with H2O.
This implies that it has a much lower dissolving and limit at – 82˚C and – 60˚C, separately. Like SO2, the presence of Sulfur implies that this atom has an impactful scent in a vaporous structure even though it is dull.
How is H2S utilized in the real world?
Hydrogen sulfide shows up in a wide range of ways inside the regular world. First of all, it is a significant constituent of the sulfur cycle. Microorganisms generally convert the Sulfur from natural components to inorganic atoms like H2S.
Hydrogen sulfide’s principle utilization is a capacity compound that can be changed over to unadulterated Sulfur during responses to shape a wide range of sulfur-containing compounds. Hydrogen sulfide might have additionally made a mass eradication due to its development inside the environment.
In light of this reality, it is easy to envision the poisonousness of hydrogen sulfide towards life structures like individuals. It adversely impacts the appropriate sensory system working fundamentally even though it will influence other body frameworks too.
There are sure creatures adjusted to living in high-H2S conditions because those conditions exist in deep submerged volcanic ocean vents.
Frequently asked questions
Here is some frequenty asked questions related to the article H2S polar or nonpolar
Is H2S Polar or nonpolar?
H2S is a somewhat polar particle due to the little distinction in electronegativity upsides of Hydrogen (2.2) and Sulfur (2.58) molecules.
Why is h2o polar but not H2S?
In Water, the oxygen iota is exceptionally electronegative and can spellbind (somewhat) the hydrogen particles; accordingly, hydrogen-hydrogen connections between the H2O atoms can be framed, making an extremely high edge of boiling over. In H2S, those bonds don’t exist since Sulfur is substantially less electronegative.
What type does bonding is H2S?
Hydrogen sulfide (H2S) is a covalent compound because the bond structures between two hydrogens and one Sulfur are covalent. The covalent bond is framed because of the sharing of an electron between hydrogen and sulfur molecules to finish their octet shell and thus, achieve security.
Is H2S straight or bend?
H2S is a non-straight atom. The bond point between the two H-S bonds is around 92%. Every two 3p-orbitals of sulfur-containing one electron can cover with 1s orbitals of hydrogen particles.
What is the electronegativity difference of H2S?
The distinction in electronegativities of Hydrogen (2.20) and Sulfur (2.58) is as old as among Hydrogen and carbon (2.55). The C-H bond is seen as nonpolar; thus, should the H-S bond along these lines.
Conclusion
H2S Polar or Nonpolar Hydrogen sulfide (H2S) is nonpolar because of its nonpolar H-S bonds. The EN distinction between Hydrogen and Sulfur is 0.4, so hydrogen and sulfur structure nonpolar bonds. H2S is the synthetic equation of the compound hydrogen sulfide.
Hydrogen sulfide is a covalent compound comprised of 2 hydrogen particles clung to a focal sulfur iota. Like Water (H20), hydrogen sulfide is a hydrogen chalcogenide, a compound made out of Hydrogen and a gathering of 16 components (Oxygen, Sulfur, selenium, tellurium.
https://howtodiscuss.com/t/net-worth-percentile-calculator/119666