Hydrogen sulfide lewis structure
The Lewis structure of hydrogen sulfide is easy to draw and understand. In this compound, both hydrogen atoms require electrons to form a covalent-sulfur bond. H2S’s Lewis structure is similar to H2S. Sulfur requires eight electrons to meet the octet law requirements. However, since hydrogen is a member of group 1, it only needs one electron to be stable.
H2s lewis structure
Place the sulfur atom in the center and align the valence electrons around it.
Two hydrogen atoms are now placed on either side of the central atom.
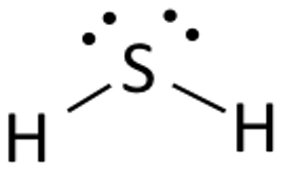
H2s lewis structure molecular geometry
H2S or hydrogen sulfide gas is colorless in nature. Sour gas, sewage gas, etc. Like many other pet names, this gas is also poisonous and corrosive.
I’m sure you didn’t expect this gas to smell good! Yes, you’re right, hydrogen sulfide gas smells like rotten eggs !!
The molar mass of H2S is 34.08 g / mol and its density is 1.363 g dm-3. The melting and boiling points of H2S are -82 ° C and -60 ° C, respectively.
H2S has a covalent bond. Sharing 2 electrons with 2 hydrogen atoms creates a covalent bond as the sulfur atom is completely filled.
Now, to understand this, we need to know the steps to draw the lewis structure in the first place.
First of all, it is important to determine the number of valence electrons present in a compound.
In this valence compound, the electrons are as follows:
Valence electrons of hydrogen = 1
2 * hydrogen atom = 2
Valence electrons of sulfur = 6
Therefore, total valence electrons = 2 + 6 = 8.
The molecular geometry of H2s is bent.
Dot structure h2s
The h2s dot structure can be drawn in 4 steps:
Step 1:
To draw the Lewis point structure of H₂S, we first need to find the valence electrons of sulfur and hydrogen. We express valence electrons as points in the Lewis point structure.
To get the valence electrons of sulfur, we need to look at the electron configuration of sulfur.
S (16) = 1s22s22p⁶3s23p⁴
The maximum value of the principal quantum number here is n = 3.
The maximum value of the principal quantum number n indicates the valence shell and we know that the electron in the valence shell is called the valence shell.
The nun of the valence electrons in sulfur is 6.
Again, we need to find the valence electrons of hydrogen H.
To get the valence electrons of hydrogen, we need to look at the electron diagram of hydrogen.
H (1) = 1 second
The maximum value of the principal quantum number here is n = 1.
The maximum value of the principal quantum number n indicates the valence shell and we know that the electron in the valence shell is called the valence shell.
The number of valence electrons in hydrogen is 1.
Step 2: H2S Valence Electrons
Now we need to count the total valence electrons of H2S.
S = 6
2 × H = 1 × 2 = 2
Therefore, the total valence electrons for the molecular valence H2S are eight (6 + 2 = 8).
Installation procedure for H2S Lewis-3 structure:
Now we need to define the central atom in H₂S. The central atom is that type of atom that is a single atom or has a lower electronegativity. In the case of H₂S, S is the central atom and H is the outer atom. Hydrogen is always an outer atom.
Installation procedure for H2S Lewis-4 structure:
Now we need to connect the outer atom to the central atom by using a dot or line (-) every line representing a point 【●●】, two valence electrons.
Is h2s a polar molecule?
H2S is a slightly polar molecule due to its geometric structure, curvature shape, and the slight difference between the electronegativity of hydrogen (2.2) and sulfur (2.58), resulting in a dipole moment at zero Another property of H2S is that it easily reacts with metal ions to result in the formation of metal sulfides.
Bonding in h2s
If the atoms have very similar electronegativity, they form a non-polar covalent bond at H2S. The S atom is attached to 2 H atoms with electronegativity of H = 2.2. And S = 2.56, since the difference is not large, the resulting bond is covalent. (Polar covalent)
h2s lewis structure shape
Additionally, the electron geometry for H2S is tetrahedral as the 4 electrons that make up 2 odd pairs around the sulfur atom are arranged in a tetrahedral shape.
h2s molecular shape
The molecular form of hydrogen sulfide is curved. The central sulfur atom is bonded to two hydrogen atoms.
What is the lewis structure for h2s?
The Lewis structure of hydrogen sulfide is easy to draw and understand. In this compound, both hydrogen atoms need one electron to form a covalent-sulfur bond. H2S’s Lewis structure is similar to H2S.Sulfur requires eight electrons to meet the octet law requirements.
Dipole moment value of h2s
Since S is more electronegative than H, each S - H bond is polarized with the bond moment as shown, since H2S is a molecule that bends the vector sum of the dipole moment. The bonds will produce all dipole moments that are non-zero. Since the permanent dipole moment is NON-ZERO H2S, it will show a dipole-dipole interaction.
Besides, is hydrogen sulfide a polar or non-polar molecule? Hydrogen sulfide is not polar. Although molecular geometry allows polarity But the bonds are nonpolar, so the molecules aren’t either. The polarity is determined by the electronegativity.
h2s covalent bond
There are six electrons in the outer shell and two are needed to fill eight of them. These are both hydrogen atoms. Each hydrogen atom can share an electron with a sulfur atom and form intermolecular bonds. The result is a single molecule of hydrogen sulfide.
Does h2s have hydrogen bonding?
H2O (top) and H2S (bottom) molecules can form hydrogen bonds. Hydrogen bond is one of the reasons for water’s unique boiling and freezing behavior and other properties, but scientists have argued for decades over whether the reduction of hydrogen sulphide, water’s smelly triatom cousin. And they also have hydrogen bonds.
Hydrogen sulfide electronegativity
The electronegativity of hydrogen and sulfur is 2.20 and 2.58 respectively. Their electronegativity difference of 0.38 is less than 0.5. Therefore, H2S is a non-polar bond.
h2s valence electrons
H2S is called hydrogen sulfide. First of all we have to count the total valence electrons. We just add the valence electrons of each of the corresponding atoms.
S = 6
H = 1 x 2
8 electrons
Next, we need to define the central atom, in this case S.
Now we have to connect atoms and make bonds. We can make two S-H bonds.
S
/ \
H H
Finally, we have to distribute the rest of the electrons on S where it follows the octet law.
: S:
/ \
H H
Frequently Asked Questions (FAQ’s)
Q: Which polar water is greater or H2S?
The water has a wind blowing due to the electronegativity of Vander waal, this basin, the electric current also has a chrysalis force) and the water has a Around Tom Taew and lower bed.
Q: Does H2S have double bonds?
In an H2S molecule, two hydrogen atoms form a bond with the central sulfur atom. Two single bonds form in the molecule. These bonds use the four valence electrons and therefore the remaining four valence electrons.
Q: Why does H2S have a low boiling point?
Hydrogen sulfide is structurally similar to water. Sulfur is not as electronegative as oxygen, so hydrogen sulfide is not as polar as water. Therefore, relatively weak intermolecular forces exist for H2S, and their melting and boiling points are much lower than those for water.
Q: Why is the bond angle in H2O greater than H2S?
The bond angle of H2O is greater because oxygen is more electro-negative than sulfur, so the electron pair of O-H bond is closer to oxygen.
Conclusion
H2s is polar in nature. In h2s, one sulphur atom is placed in the center while other 2 hydrogen atoms are placed on both of the sides to form a h2s structure. H2s has a dot structure.