The main properties of water are its polarity, cohesion, adhesion, surface tension, high specific heat, and evaporative cooling.Polarity
A water molecule is slightly charged on both ends. This is because oxygen is more electronegative than hydrogen.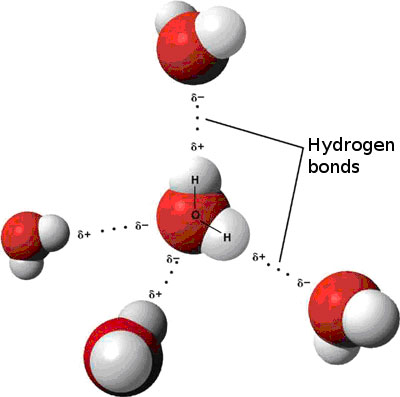 Check out video of a stream of water being bent - a plastic ruler is used in the demo. The stream of water bends due to the polarity of water molecules.
Check out video of a stream of water being bent - a plastic ruler is used in the demo. The stream of water bends due to the polarity of water molecules.
YouTube
Video from: Noel PaullerCohesion
Hydrogen bonds hold water molecules together, as seen in the picture above. Cohesion creates surface tension which is why if you fill a spoon with water, drop by drop, the water volume will actually be bigger than the spoons surface before the water falls off.Here is video showing how a paperclip can float on water - its actually being held up by the hydrogen bonds formed between water molecules which give water its surface tension.
YouTube
Video from: Noel PaullerAdhesion
Similar to cohesion, but adhesion is when the hydrogen bonds in water allow for the water molecules to be held to another subtance.High Specific Heat
Specific heat is amount of heat absorbed or lost for 1g to change 1ºC, which in the case of water, is pretty high. This allows for evaporative cooling to occur, which is when heat energy is transferred to water molecules , and evaporating water removes a lot of heat energy from an organism (eg. when we sweat)Other important characteristics of water involve it being a universal solvent, along with its unusual density. Water, unlike any other solid-liquid, is more dense in its liquid form than as a solid, which is why ice floats, and this allows for entire habitats to exist underneath layers of ice floating on oceans. Its neutral pH (7) is also a relevant characteristic.